Abstract
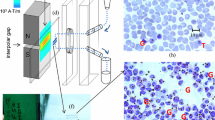
Intraerythrocytic parasites ofPlasmodium vinckei andPlasmodium berghei were separated according to their developmental stages using discontinuous Percoll gradients. Contaminating nucleated blood cells such as leukocytes were removed by elutriation centrifugation. The stages were unequivocally identified in smears using a newly developed DNA-specific staining procedure with mithrmycin and fluorescence microscopy. This stain can also be used to detect parasites in human blood of very low parasitemias. The combination of methods described has many possible applications in immunologic and biochemical parasite research.
Similar content being viewed by others
References
Eling W (1977) Ficoll fractionation for the separation of parasitized erythrocytes from malaria infected blood. Bull WHO 57: 105–114
Eugui EM, Allison AC (1979) Separation of erythrocytes infected with murine malaria parasites in metrizamide gradients. Parasitology 79: 267–275
Heidrich HG (1970) Isolation of pure inner and outer mitochondrial membranes in preparative scale. In: Advances in automated chemistry: proceedings of the international Technicon congress 1969, MEDIAD-Technicon, Tarrytown-New York pp 347–350
Heidrich HG, Rieckmann KH (1980) Free-flow electrophoresis separation of intracellular parasites (Plasmodium falciparum) from culture. Eur J Cell Biol 22: 516
Heidrich HG, Rüssmann L, Bayer B, Jung A (1979) Free-flow electrophoresis for the separation of malaria infected and non-infected mouse erythrocytes and for isolation of free parasites (Plasmodium vinckei). Z Parasitenkd 58: 151–159
Howard RJ, Battye FL (1979)Plasmodium berghei — infected red cells sorted according to DNA stain. Parasitology 78: 263–270
Hyman BC, MacInnis AJ (1979) Rapid detection of malaria and other blood stream parasites by fluorescence microscopy with 4 6 diamidino-2-phenylindole (DAP). J Parasitol 65: 421–425
Kilejian A (1980) Stage-specific proteins and glycoproteins ofPlasmodium falciparum: identification of antigens unique to schizonts and merozoites. Proc Natl Acad Sci USA 77: 3695–3699
Lunde MM, Powers KS (1976) The preparation of malaria hemagglutination antigen. Ann Trop Med Parasitol 70: 283–291
McAllister RO, Gordon DM (1976) Schizont-infected cell enrichment in rodent malaria. J Parasitol 62: 664–669
McEven CR, Juhos ET, Stallard RW, Schnell JV, Siddiqui WA, Geimann QM (1971) Centrifugal elutriation for the removal of leukocytes from malaria-infected monkey blood. J Parasitol 57: 887–890
Miller LH, Chien S (1971) Density distribution of red cells infected byPlasmodium knowlesi andPlasmodium coatneyi. Exp Parasitol 29: 451–456
Mrema JE, Campbell GH, Miranda R, Jamarillo AL, Rieckmann KH (1979) Concentration and separation of erythrocytes infected withPlasmodium falciparum by gradient centrifugation. Bull WHO 57: 133–138
Pasvol G, Wilson RJ, Smalley ME, Brown J (1978) Separation of viable schizont-infected red cells ofPlasmodium falciparum from human blood. Ann Trop Med Parasitol 72: 87–88
Pertoft H, Laurent TC (1979) Isopycnic separation of cells and cell organelles by centrifugation in modified colloidal silica gradients. In: Catsimpolas N (ed) Methods of cell separation. Plenum, New York, pp 25–56
Reese RT, Langreth SG, Trager W (1979) Isolation of stages of the human parasitePlasmodium falciparum from culture and from blood. Bull WHO 57: 53–61
Rowley PT, Siddiqui WA, Geiman OM (1967) Separation of malarial parasites according to age by density gradient centrifugation. J Lab Clin Med 70: 933–937
Sanderson RJ, Bird KE (1977) Cell separation by counterflow centrifugation. Methods Cell Biol 15: 1–14
Siddiqui WA, Kan SC, Kramer K, Richmond-Crum SM (1979) In vitro production and partial purification ofPlasmodium falciparum antigen. Bull WHO 57: 75–82
Sodeman TM (1970) The use of fluorochromes for the detection of malaria parasites. Am J Trop Med Hyp 19: 40–45
Tosta CE, Sedegah M, Henderson DC, Wedderburn N (1980)Plasmodium yoelii andPlasmodium berghei: isolation of infected erythrocytes from blood by colloidal silica gradient centrifugation. Exp Parasitol 50: 7–15
Ward DC, Reich E, Goldberg IH (1965) Base specificity in the interaction of polynucleotides with antibiotic drugs. Science 149: 1259–1263
Williamson J, Cover B (1966) Separation of blood-cell-free trypanosomes and malaria parasites on a sucrose gradient. Trans R Soc Trop Med Hyg 60: 425–427
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rüssmann, L., Jung, A. & Heidrich, HG. The use of percoll gradients, elutriator rotor elution, and mithramycin staining for the isolation and identification of intraerythrocytic stages ofPlasmodium berghei . Z. Parasitenkd. 66, 273–280 (1982). https://doi.org/10.1007/BF00925344
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00925344