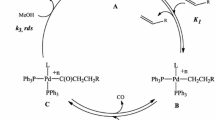
Conclusions
-
1.
The insertion into cycloheptanone of a methyl in either the 3 or 4 position, and also of two methyls in the 3 and 6 positions, does not affect the rate of hydrogen transfer from 2-propanol to the cycloheptanones in the presence of the triphenylphosphine complexes of Rh and Ru.
-
2.
The presence of geminal dimethyl groupings in the 3 and 6 positions lowers the reduction rate of the cycloheptanones, which is explained by a shielding of the carbonyl by the axial methyls.
-
3.
Conformational analysis revealed that in principle the cycloheptanone homologs differ from the homologs with smaller rings: transition of the axial substituent to the equatorial can occur without the complete inversion of the seven-membered ring. Both stereoisomers of 3, 6-dimethylcycloheptanone can exist in the equatorial form. This is in good agreement with the experimental data on the reduction rates.
Similar content being viewed by others
Literature cited
V. Z. Sharf, L. Kh. Freidlin, and V. N. Krutii, Izv. Akad. Nauk SSSR, Ser. Khim., 2264 (1973).
L. Kh. Freidlin, V. Z. Sharf, V. N. Krutii, and I. S. Shekoyan, Izv. Akad. Nauk SSSR, Ser. Khim., 1330 (1974).
J. A. Osborn, F. H. Jardine, J. E. Young, and G. Wilkinson, J. Chem. Soc., A, 1711 (1966).
T. A. Stephenson and G. Wilkinson, J. Inorg. Nucl. Chem.,A28, 945 (1966).
A. L. Liberman and T. V. Vasina, Dokl. Akad. Nauk SSSR,198, 1344 (1971).
A. L. Liberman and T. V. Vasina, Zh. Organ. Khim.,3, 690 (1967).
D. B. Furman, S. A. Chelmakova, T. V. Vasina, and A. L. Liberman, Izv. Akad. Nauk SSSR, Ser. Khim., 2124 (1974).
Y. Sasson, J. Blum, and E. Dunkelblum, Tetrahedron Lett., 3199 (1973).
V. Z. Sharf, L. Kh. Freidlin, V. N. Krutii, and T. V. Lysyak, Izv. Akad. Nauk SSSR, Ser. Khim., 2195 (1975).
N. L. Allinger, J. Am. Chem. Soc.,81, 5727 (1959).
A. L. Liberman and T. V. Vasina, Izv. Akad. Nauk SSSR, Ser. Khim., 854 (1975).
J. B. Hendrickson, J. Am. Chem. Soc.,83, 4537 (1961).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 5, pp. 1111–1115, May, 1975.
Rights and permissions
About this article
Cite this article
Krutii, V.N., Chelmakova, S.A., Gurovets, A.S. et al. Conformation effects in reduction of methyl-substituted cyclopentanones with 2-propanol in presence of RhCl(PPh3)3 or RuCl2(PPh3)3 . Russ Chem Bull 24, 1019–1022 (1975). https://doi.org/10.1007/BF00922956
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00922956