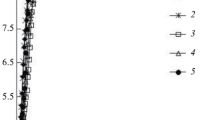Conclusions
-
1.
Effective rate constants (kef) for the hydrolysis of ethyl isovalerate in aqueous solutions containing from 4.95 to 41.3 mass % HCl, or from 2.52 to 94.0 mass % H2SO4, have been determined spectrophotometrically at 25°C.
-
2.
The quantity kef increases with increasing acid concentration in aqueous HCl solutions. In aqueous sulfuric acid solutions, kef passes through a maximum at 49.8–55.9% H2SO4 and a minimum at 74.9% H2SO4.
-
3.
In the hydrochloric acid and sulfuric acid solutions in question here (up to 74.9% H2SO4), the hydrolysis of ethyl isovalerate proceeds through an activated complex containing one un-ionized ester molecule and one hydronium ion carrying at least one solvation water molecule. The water molecule functions here as a nucleophilic agent.
-
4.
The data obtained can be interpreted quantitatively by assuming that ionizatioa results in the formation of a protonized form of the ester and an unreactive complex in which the carbonyl group oxygen is bound to the hydronium ion.
-
5.
Hydrolysis proceeds through the protonized form of the ester in sulfuric acid solutions with concentrations in excess of 74.9%.
Similar content being viewed by others
Literature cited
M. I. Vinnik and N. B. Librovich, Izv. Akad. Nauk SSSR, Ser. Khim., 2211 (1975).
N. B. Librovich and M. I. Vinnik, Zh. Fiz. Khim.,50, 150 (1976).
M. I. Vinnik, L. R. Andreeva, and I. M. Medvetskaya, Izv. Akad. Nauk SSSR, Ser. Khim., 540 (1976).
M. I. Vinnik, Izv. Akad. Nauk SSSR, Ser. Khim., 998 (1973).
Dictionary of Organic Compounds, Vol. 2, London (1946).
N. G. Zarakhani, N. B. Librovich, and M. I. Vinnik, Zh. Fiz. Khim.,45, 1733 (1971).
M. I. Vinnik, Uspekhi Khimii,35, 1922 (1966).
W. F. Jiauque, E. W. Hornung, J. E. Kunzler, and T. R. Rubin, J. Amer. Chem. Soc.,82, 62 (1960).
K. Yates and R. A. McClelland, J. Amer. Chem. Soc.,89, 2686 (1967).
V. D. Maiorov and N. B. Librovich, Zh. Fiz. Khim.,47, 2298 (1973).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 7, pp. 1480–1485, July, 1976.
Rights and permissions
About this article
Cite this article
Vinnik, M.I., Librovich, N.B. Hydrolysis of ethyl isovalerate in aqueous hydrochloric and sulfuric acid solutions. Russ Chem Bull 25, 1415–1419 (1976). https://doi.org/10.1007/BF00920808
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00920808