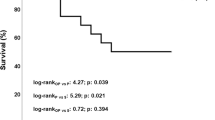
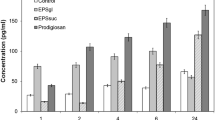
Abstract
Rats given a single intraperitoneal injection of an aqueous suspension of peptidoglycan-polysaccharide polymers derived from group A streptococcal cell wall (PG-APS) develop a severe, chronic, erosive arthritis which resembles human rheumatoid arthritis. The treatment of PG-APS-mjected rats with a single intravenous injection of 0.4 mg of mutanolysin prevents the development of chronic arthritis, even when administration of the enzyme is delayed until severe acute arthritis has developed. PG-APS activates complement both in vitro and in vivo. Digestion of PG-APS with mutanolysin in vitro destroys the ability to activate both the alternate and classical pathways of human serum complement, and the loss of complement activation parallels the extent of PG-APS degradation. There is also a reduction in the in vivo complexing of C3 with PG-APS in the limbs of PG-APS-injected rats treated with mutanolysin, compared to control rats injected with PG-APS and treated with phosphate-buffered saline. This association between loss of arthropathic activity and loss of activation of complement is consistent with the hypothesis that activated complement products form a part of the inflammatory mediators involved in the acute and chronic phases of bacterial cell wall-induced arthritis. This may also partially explain how mutanolysin treatment alleviates cell wall-induced arthritis in the rat.
Similar content being viewed by others
References
Cromartie, W. J., J. G. Craddock, J. H. Schwab, S. K. Anderle, andC. Yang, 1977. Arthritis in rats after systemic injection of streptococcal cells or cell walls.J. Exp. Med. 146:1585–1602.
Dalldorf, F. G., W. J. Cromartie, S. K. Anderle, R. L. Clark, andJ. H. Schwab. 1980. The relation of experimental arthritis to the distribution of streptococcal cell wall fragments.Am. J. Pathol. 100:383–402.
Clark, R. L., J. T. Cuttino, S. K. Anderle, W. J. Cromartie, andJ. H. Schwab. 1979. Radiologic analysis of arthritis in rats after systemic injection of streptococcal ceil walls.Arthritis Rheum. 22:25–35.
Spitznagel, J. K., K. J. Goodrum, andD. J. Warejcka. 1983. Rat arthritis due to whole group B streptococci. Clinical and histopathologic features compared with groups A and D.Am. J. Pathol. 112:37–47.
Wilder, R. L., G. B. Calandra, A. J. Garvin, K. D. Wright, andC. T. Hansen. 1982. Strain and sex variation in the susceptibility to streptococcal cell wall-induced polyarthritis in the rat.Arthritis Rheum. 25:1064–1072.
Fox, A., R. R. Brown, S. K. Anderle, C. Chetty, W. J. Cromartie, H. Gooder, andJ. H. Schwab. 1982. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls.Infect. Immun. 35:1003–1010.
Chetty, C., D. G. Klapper, andJ. H. Schwab. 1982. Soluble peptiodoglycan-polysaccharide fragments of the bacterial cell wall induce acute inflammation.Infect. Immun. 38:1010–1119.
Janusz, M. I., C. Chetty, R. A. Eisenberg, W. J. Cromartie, andJ. H. Schwab, 1984. Treatment of experimental erosive arthritis in rats by injection of the muralytic enzyme mutanolysin.J. Exp. Med. 160:1360–1374.
Greenblatt, J., R. J. Boackle, andJ. H. Schwab, 1978. Activation of the alternate complement pathway by pepetidoglycan from streptococcal cell wall.Infect. Immun. 19:296–303.
Forgren, A., andP. G. Quie. 1974. Influence of the alternate complement pathway on opsonization of several bacterial species.Infect. Immun. 10:402–404.
Kotani, S., H. Takada, M. Tsujimoto, T. Ogawa, K. Kato, T. Ohunaga, Y. Ishihama, A. Kawasaki, I. Morisali, N. Kono, T. Shimono, T. Shiba, S. Kusumoto, M. Image, K. Harada, T. Kitaura, S. Kano, S. Inai, K. Nagai, M. Matsumoto, T. Kubo, M. Kato, Z. Tada, K. Yokogawa, S. Kawata, andA. Inoul. 1981. Immunomodulating and related biological activities of bacterial cell walls and their components, enzymatically prepared or synthesized.In Immunomodulation by Bacteria and Their Products. H. Friedman, T. W. Klein, and A. Szentivanyi, editors. Plenum Press, New York. 231–273.
McBride, W. H., P. M. Weir, A. B. Kay, P. Pearce, andJ. R. Caldwell. 1975. Activation of the classical and alternative pathways of complement byC. parvum.Clin. Exp. Immunol. 19:143–147.
Tauber, J. W., M. J. Polley, andJ. B. Zabriskie. 1976. Non specific complement activation by streptococcal structures II. Properdin-independent initiation of the alternate pathway.J. Exp. Med. 143:1352–1366.
Winklestein, J., andA. Tomasz. 1977. Activation of the alternative pathway by pneumocoiccal cell wall.J. Immunol. 118:451–454.
Lambris, J. D., J. B. Allen, andJ. H. Schwab. 1982. In vivo changes in complement induced with peptidoglycan-polysaccharide polymers from streptococcal cell walls.Infect. Immun. 35:377–380.
Schwab, J. H., J. B. Allen, S. K. Anderle, F. Dalldorf, R. Eisenberg, andW. J. Cromartie. 1982. Relationships of complement to experimental arthritis induced in rats with streptococcal cell walls.Immunology 46:83–88.
Kotani, S. A. Kawasaki, S. Inai, K. Nagaki, M. Matsumoto, T. Shiba, S. Kusumoto, M. Inage, K. Yokogawa, S. Kawata, andA. Inoue. 1980. Activation of human complement system by enzymatic digests of bacterial cell wall peptidoglycans and 6-O-acyl-muramyl peptides.Int. J. Immunopharmacol. 2:213.
Siegel, J., S. Hurst, E. Libermann, S. Coleman, andA. Bleiweis. 1981. Mutanolysininduced spenoplasts ofStreptococcus mutans are true protoplasts.Infect. Immun. 31:808–815.
Stimpson, S. A., R. R. Brown, D. G. Klapper, W. J. Cromartie, andJ. H. Schwab. 1986. Arthropathic properties of cell wall polymers from normal flora bacteria.Infect Immun. 51:240–249.
Dische, Z., andL. B. Shettles. 1948. A specific color reaction of methyl pentose and a spectrophotometric micromethod for their determination.J. Biol. Chem. 175:595–603.
Sawardeker, J. S., J. H. Sloneker, andA. Jeanes. 1965. Quantitative determination of monosaccharides as their alditol acetates by gas-liquid chromatography.Anal. Chem. 37:1602–1604.
Klapper, D. G. 1982. A new low cost, fully automated amino acid analyzer using a gradient HPLC.In Methods in Protein Sequence Analyses. M. Elzinga, editor. Humana Press, Clifton, New Jersey. 509–515.
Platts-Mills, T.A.E., andK. Ishizaka. 1974. Activation of the alternative pathway of human complement of rabbit cells.J. Immunol. 113:348–358.
Keystone, E. C., H. V. Schlorlemmer, C. Pope, andA. C. Allison. 1977. Zymosan-induced arthritis. A model of chronic poliferative arthritis following activation of the alternative pathway of complement.Arthritis Rheum. 20:1396–1401.
Schlorlemmer, H. V., H. V. Bitter-Suermann, andA. C. Allison 1977. Complement activation by the alternative pathway and macrophage enzyme secretion in the pathogenesis of chronic inflammation.Immunology 32:929–940.
Ruddy, S., andK. F. Austen. 1970. The complement system in rheumatoid synovitis. I. An analysis of complement component activities in rheumatoid synovial fluids.Arthritis Rheum. 13:713–723.
Hugli, T. E. 1981. The structural basis for anaphylatoxin and chemotactic functions of C3a, C4a, and C5a anaphylatoxins.Crit. Rev. Immunol. 1:321–366.
Hugli, T. E., andE. J. Muller-Eberhard. 1978. Anaphylatoxins: C3a and C5a.Adv. Immunol. 26:1–53.
Snyderman, R., andE. J. Goetzl. 1981. Molecular and cellular mechanisms of leukocyte chemotaxis.Science 213:830–837.
Ward, P. A., andL. J. Newman, 1969. A neutrophil chemotactic factor from human C5a.J. Immunol. 102:93–99.
Fernandez, H. N., P. N. Henson, A. Otani, andT. E. Hugli. 1978. Chemotactic responses to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under simulated in vivo conditions.J. Immunol. 120:109–115.
Larsen, G. L., andP. M. Henson. 1983. Mediators of Inflammation.In Annual Review of Immunology. W. Paul, C. G. Fathman, and H. Metzger, editors. Annual Reviews, Inc., Palo Alto, California.
Regal, J. F., andR. J. Pickering. 1981. C5a-induced tracheal contraction: Effect on an SRS-A antagonist and inhibitors of arachidonate metabolism.J. Immunol. 126:313–316.
Stimler, N. P., M. K. Bach, C. M. Bloor, andT. Hugli. 1982. Release of leukotrienes from guinea pig lung stimulated by C5a des arg anaphylatoxin.J. Immunol. 128:2247–2252.
Stimler, N. P., W. E. Brocklehurst, C. M. Bloor, andT. Hugli. 1981. Anaphylatoxinmediated contraction of guinea pig lung strips: A nonhistamine tissue response.J. Immunol. 126:2258–2261.
Chenoweth, D. E., M. G. Goodman, andW. O. Weigle. 1982. Demonstration of a specific receptor for human C5a anaphylatoxin on murine macrophages.J. Exp. Med. 156:68–78.
Scrouzer, C. A., W. A. Scott, A. L. Hamill, andZ. A. Cohn. 1982. Synthesis of leukotriene C and other arachidonic acid metabolites by mouse pulmonary macrophages.J. Exp. Med. 155:720–733.
Fearon, D. T., andW. Wong. 1983. Complement ligand-receptor interactions that mediate biological responses.In Annual Review of Immunology, W. Paul, C. G., Fathman, andH. Metzger, editors. Annual Reviews, Inc., Palo Alto, California. 243–271.
Dahlen, S.-E., J. Bjork, P. Hedquist, K.-E. Arfors, S. Hammarstrom, J.-A. Lindgren, andB. Samuelsson. 1981. Leukotrienes promote plasma leakage and leukocyte adhesion in postcapillary venules: In vivo effects with relevance to the acute inflammatory response.Proc. Natl. Acad Sci. U.S.A. 78:3887–3891.
Allison, A. C. 1984. Role of macrophage activation in the pathogenesis of chronic inflammation and its pharmalogical control.In Advances in Inflammation Research, Vol. 7. 1. Overness, R. Capetola, and S. Wong, editors. Raven Press, New York, 201–221.
Schorlemmer, H. V., andA. C. Allison. 1976. Effects of activated complement components on enzyme secretion by macrophages.Immunology 31:781–788.
McCarthy, K., andP. M. Henson. 1979. Induction of lysosomal enzyme secretion by alveolar macrophages in response to the purified complement fragments C5a and C5a des-arg.J. Immunol. 123:2511–2517.
Leong, P. A., J. H. Hwab, andM. S. Cohen. 1984. Interaction of group A streptococcal peptidoglycan polysaccharide with human polymorphonuclear leukocytes: Implications for pathogenesis of chronic inflammation.Infect. Immun. 45:160–165.
Pryzwansky, K. B., E. K. MaCrae, andJ. D. Lambris. 1983. Capping of complement receptors on human neutrophils induced by group A streptococcal cell walls.J. Immunol. 130:1674–1677.
Goldstein, I., D. Roos, H. B. Kaplan, andG. Weissman. 1975. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis.J. Clin. Invest. 56:1155–1163.
Burger, R., V. Hadding, H. V. Schlorlemmer, V. Brade, andD. Bitter-Suermann. 1975. Dextran sulfate: A synthetic activator of C3 via the alternate pathway. I. Influence of molecular size and degree of sulfation on the activation potency.Immunology 29:549–554.
Janusz, M. J., R. E. Esser, andJ. H. Schwab. 1986. In vivo degradation of bacterial cell wall by the muralytic enzyme mutanolysin.Infect. Immun. 52:459–467.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Janusz, M.J., Eisenberg, R.A. & Schwab, J.H. Effect of muralytic enzyme degradation of streptococcal cell wall on complement activation in vivo and in vitro. Inflammation 11, 73–85 (1987). https://doi.org/10.1007/BF00917773
Issue Date:
DOI: https://doi.org/10.1007/BF00917773