Summary
-
1.
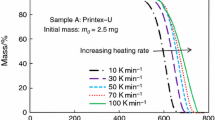
Adsorption isotherms have been determined and compared for the vapors of water, methanol, and benzene on a number of carbon blacks which had been subjected to different thermal treatments. With increasing treatment temperature, there were decreases in both the amount of surface oxygen, capable of exchange reaction with aqueous NaOH, and in surface roughness of the blacks. The surface of the blacks became much more uniform after treatment in a hydrogen stream at 1700°.
-
2.
Adsorption of water vapor fell rapidly with increasing graphitization, mainly as the result of removal of surface oxides. With blacks graphitized in a hydrogen stream at 1700°, water vapor adsorption was very small, even in the region of high pressure ratios.
-
3.
Adsorption of methanol vapor on graphitized blacks dropped markedly in the region of low pressure ratios, as the result of destruction of surface oxides. In this case, decrease in surface roughness played a secondary role.
-
4.
Adsorption of benzene vapor on graphitized blacks decreased, with decreasing surface roughness and surface coverage with chemical compounds, in the region of low surface coverage.
-
5.
The surface properties of blacks of different origin were very similar after graphitization in a hydrogen stream at 1700δ.
Similar content being viewed by others
Literature cited
B. E. Warren, J. Chem. Phys. 2, 551 (1934); J. Biscoe and B. E. Warren, J. Appl. Phys. 13, 364 (1942).
G. L. Clark, A. G. Eckert, and B. L. Burton, Industr. and Engng. Chem. 41, 201 (1949).
W. D. Schaeffer, W. R. Smith, and M. H. Polley, Industr. and Engng. Chem. 45, 1721 (1953).
M. H. Polley, W. D. Schaeffer, and W. R. Smith, J. Phys. Chem. 57, 469 (1953).
R. A. Beebe, J. Biscoe, W. R. Smith, and C. B. Wendell, J. Am. Chem. Soc. 69, 95 (1947).
R. B. Anderson and P. H. Emmett, J. Appl. Phys. 19, 367 (1948).
D. S. Villars, J. Am. Chem. Soc. 69, 214 (1947); 70. 3655 (1948).
R. S. Stearns and B. L. Johnson, Industr. and Engng. Chem. 43, 146 (1953).
G. Kraus, J. Phys. Chem. 59, 343 (1955).
N. A. Shilov, E. G. Shatunskaya, and K. V. Chmutov, Z. Phys. Chem. A 148, 233 (1930); A 149, 211 (1930); A 150, 31 (1930).
M. M. Dubinin and E. D. Zaverina, J. Phys. Chem. 13, 151 (1939); Bull. Acad. Sci. USSR, Div. Chem. Sci. 594 (1955)s* E. D. Zaverina and M. M. Dubinin, J. Phys. Chem. 21, 1373 (1947).
M. M. Dubinin, Surface Chemical Compounds and Their Role in Adsorption Phenomena, pub. MGU (1957), p. 9.
C. Pierce and R. N. Smith, J. Phys. Chem. 58, 298 (1954).
R. N. Smith, J. Duffield, R. Pierotti, and Y. Mooi, J. Phys. Chem. 60, 459 (1956).
S. Ross and W. W. Pultz, J. Coll. Sci. 13, 397 (1958).
R. A. Beebe and R. M. Dell, J. Phys. Chem. 59, 746 (1955).
G. D. Halsey, J. Am. Chem. Soc. 73, 2693 (1951); 74, 1082 (1952).
A. V. Kiselev and E. V. Khrapova, Bull. Acad. Sci. USSR, Div. Chem. Sci. 389 (1958).
R. A. Beebe and D. M. Young, J. Phys. Chem. 58, 95 (1954).
C. H. Amberg, W. B. Spenser, and R. A. Beebe, Canad. J. Chem. 33, 305 (1955).
R. A. Beebe, M. H. Polley, W. R. Smith, and C. B. Wendell, J. Am. Chem. Soc. 69, 2294 (1947).
J. W. Ross and R. J. Good, J. Phys. Chem. 60, 1167 (1956).
N. N. Avgul', G. I. Berezin, A. V. Kiselev, and I. A. Lygina, J. Phys. Chem. 30, 2106 (1956).
N. N. Avgul', G. I. Berezin, A. V. Kiselev, and I. A. Lygina, Bull. Acad. Sci. USSR, Div. Chem. Sci. 1304 (1956); 1021 (1957); 787 (1959).
N. N. Avgul', Surface Chemical Compounds and Their Role in Adsorption Phenomena, Pub, MGU (1957), p. 34.
P. H. Emmett and R. B. Andersen, J. Am. Chem. Soc. 67, 1492 (1945).
C. Pierce and R. N. Smith, J. Phys. Coll. Chem. 54, 795 (1950).
C. Pierce, R. N. Smith, J. W. Willey, and H. Cordes, J. Am. Chem. Soc. 73, 4551 (1951).
N. N. Avgul', O. M. Dzhigit, and A. V. Kiselev, Proc. Acad. Sci. USSR 86, 95 (1952).
N. N. Avgul', O. M. Dzhigit, A. V. Kiselev, and K. D. Shcherbakova, Proc. Acad. Sci. USSR 92, 105 (1953).
B. Millard, E. G. Gaswell, E. E. Leger, and D. R. Mills, J. Phys. Chem. 59, 976 (1955).
G. J. Young, J. J. Chessick, F. H. Healey, and A. C. Zettlemoyer, J. Phys. Chem. 58, 313 (1954).
A. V. Kiselev and N. V. Kovaleva, J. Phys. Chem. 30, 2775 (1956).
A. V. Kiselev, N. V. Kovaleva, V. A. Sinitsin, and E. V. Khrapova, Colloid J. 20, 444 (1958).
N. N. Avgul', O. M. Dzhigit, A. V. Kiselev, and K. D. Shcherbakova, Proc. Acad. Sci. USSR 92, 1185 (1953).
C. Pierce and R. N. Smith, J. Phys. Coll. Chem. 54, 374 (1950).
B. Millard, R. A. Beebe, and J. Cynarsky, J. Phys. Chem. 58, 468 (1954).
P. A. Tessner and M. M. Polyakova, Proc. Acad. Sci. USSR 93, 855 (1953).
R. B. Anderson and P. H. Emmett, J. Phys. Chem. 56, 756 (1952).
R. M. Dell and R. A. Beebe, J. Phys. Chem. 59, 754 (1955).
V. P. Dreving, A. V. Kiselev, and Yu. A. El'tekov, Proc. Acad. Sci. USSR 86, 95 (1952).
H. L. McDermot, and L. C. Arnell, J. Phys. Chem. 58, 492 (1954).
N. N. Avgul', G. I. Berezin, A. V. Kiselev, I. A. Lygina, and G. G. Muttik, J. Phys. Chem. 31, 1111 (1957).
A. V. Kiselev, Proc. Acad. Sci. USSR 106, 1046 (1956).
N. Smith, C. Pierce, and H. Cordes, J. Am. Chem. Soc. 72, 5595 (1950).
Author information
Authors and Affiliations
Additional information
The author's thanks are due to M. M. Dubinin for his advice and encouragement.
Rights and permissions
About this article
Cite this article
Kiselev, A.V., Kovaleva, N.V. Effect of thermal treatment of various carbons on the adsorption of vapors. Russ Chem Bull 8, 955–964 (1959). https://doi.org/10.1007/BF00916659
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00916659