Abstract
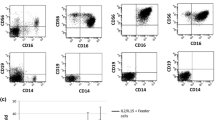
Cell lines were established from purified large granular lymphocites (LGL) isolated from the peripheral blood of seven patients with phenotypically homogeneous LGL expansions. LGL were stimulated with phytohemagglutinin (PHA) or recombinant interleukin-2 (rIL-2) and further expandedin vitro in IL-2-containing media. The surface phenotype of LGL, as assessed by monoclonal antibody staining, was T3+ T8+ in five patients, T3− T8− in one, and T3+ T8− in another patient. The cells also expressed Leu 7, Leu 11, and/or OKM 1 markers in various proportions and were identifiable as LGL by their morphological and cytochemical features. The original surface phenotype of the unstimulated LGL was retained in the IL-2-dependent cell lines from each individual patient, i.e., T3+ T8+ cells originated T3+ T8+ cell lines and T3− T8− cells originated T3− T8− cell lines. Other markers, such as Leu 11 and OKM 1, were generally lost in culture. LGL proliferated in response to rIL-2 but did not express detectable IL-2 receptors, even after prolonged periods of culture. All cell lines from each individual patient had the same surface phenotype, and within the single lines, all of the cells expressed the same markers. Cell lines from two patients consistently displayed chromosomal abnormalities. Although different in the two patients, the abnormalities were identical in all of the lines from the same patient and detectable in most of the cells examined, suggesting a clonal origin for the abnormally expanded LGL populations. Freshly isolated LGL did not exert NK activity. However, the IL-2-dependent LGL lines acquired the ability to kill K562 target cells and to produce gamma interferon (γ-IFN). No direct correlation was observed between these two properties.
Similar content being viewed by others
References
Saksela E, Timonen T, Ranki A, Hayry P: Morphological and functional characterization of isolated effector cells responsible for human natural killer activity to fetal fibroblasts and to cultured cell line targets. Immunol Rev 44:71–123, 1979
Grossi CE, Cadoni A, Zicca A, Leprini A, Ferrarini M: Large granular lymphocytes in human peripheral blood. Ultrastructure and cytochemical characterization of the granules. Blood 59:277–283, 1982
Timonen T, Ortaldo JR, Herberman RB: Characterization of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med 153:569–582, 1981
Pistoia V, Ghio R, Nocera A, Leprini A, Perata A, Ferrarini M: Large granular lymphocytes have a promoting activity on human peripheral blood erythroid burst forming units. Blood 65:464–472, 1985
Linch DC, Lipson JM, Nathan DG: Identification of three accessory cell populations in human bone marrow with erythroid burst promoting properties. J Clin Invest 75:1278–1284, 1985
Mangan DF, Hartnett ME, Matis SA, Winkelstein A, Abo T: Natural Killer cells suppress human erythroid stem cell proliferation in vitro. Blood 63:260–269, 1984
Hansson M, Beran M, Andersson B, Kiessling R: Inhibition of in vitro granulopoiesis by autologous and allogeneic human NK cells. J Immunol 129:126–134, 1982
Degli Antoni G, Perussia B, Mangoni L, Trinchieri G: Inhibition of bone marrow colony formation by human natural killer cells and by natural killer cell-derived colony inhibiting activity. J Exp Med 161:1152–1168, 1985
Scala G, Allavena P, Djeu JY, Kasahara T, Ortaldo JR, Herberman RB, Oppenheim JJ: Human large granular lymphocytes are potent producers of interleukin-1. Nature 309:56–59, 1984
Kasahara T, Djeu JY, Dougherty SF, Oppenheim JJ: Capacity of human large granular lymphocytes to produce multiple lymphokines: Interleukin-2, interferon and colony stimulating factor. J Immunol 131:2379–2383, 1983
Pistoia V, Cozzolino F, Torcia M, Castigli E, Ferrarini M: Production of B cell growth factor by a Leu 7+, OKM1+ non-T cell with the features of large granular lymphocytes (LGL). J Immunol 134:3179–3184, 1985
Degli Antoni G, Murphy M, Kobayashi M, Francis MK, Perussia B, Trinchieri G: Natural Killer (NK)-derived hematopoietic colony-inhibiting activity and NK cytotoxic factor. Relationship with tumor necrosis factor and synergism with immune interferon. J Exp Med 162:1512–1530, 1985
Ortaldo JR, Sharrow SO, Timonen T, Herberman RB: Determination of surface antigens on highly purified human NK cells by flow cytometry and monoclonal antibodies. J Immunol 127:2401–2409, 1981
Perussia B, Starr S, Abraham S, Fanning V, Trinchieri G: Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking FcR functions. I. Characterization of the lymphocyte subset reactive with B73.1. J Immunol 150:2133–2141, 1983
Lanier LL, Le AM, Phillips JH, Warner NL, Babcock GF: Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu 11 (NK-15) antigens. J Immunol 131:1789–1796, 1983
Timonen T, Ortaldo JR, Stadler BM, Bonnard GD, Sharrow SO, Herberman RB: Cultures of purified human natural killer cells: Growth in the presence of interleukin 2. Cell Immunol 72:178–185, 1982
Hercend T, Reinherz EL, Meuer S, Schlossman SF, Ritz J: Phenotypic and functional heterogeneity of human cloned natural killer cell lines. Nature 301:158–160, 1983
van der Griend RJ, van Krimpen BA, Ronteltap CPM, Bolhuis RLH: Rapidly expanded activated human killer (AK) cell clones have strong antitumor cell activity and have the surface phenotype of either T γ, T-non γ or null cells. J Immunol 132:3185–3191, 1984
Newland AC, Catovsky D, Linch D, Cawley JC, Beverley P, San Miguel JF, Gordon-Smith EC, Blecher TE, Shahriari S, Varadi S: Chronic T cell lymphocytosis: A review of 21 cases. Br J Haematol 58:433–446, 1984
Reynolds CW, Foon KA: T-γ lymphoproliferative disease and related disorders in human and experimental animals: A review of the clinical, cellular and functional characteristics. Blood 64:1146–1158, 1984
Aisenberg A, Krontiris TG, Mak TW, Wilkes BM: Rear-rangement of the gene for the beta chain of the T-cell receptor in T-cell chronic lymphocytic leukemia and related disorders. N Engl J Med 313:529–533, 1985
Bertness V, Kirsch I, Hollis G, Johnson B, Bunn PA Jr: T-cell receptor gene rearrangements as clinical markers of human T-cell lymphomas. N Engl J Med 313:534–538, 1985
Waldmann TA, Davis MM, Bongiovanni KF, Korsmeyer SJ: Rearrangements of genes for the antigen receptor on T cells as markers of lineage and clonality in human lymphoid neoplasms. N Engl J Med 313:776–783, 1985
O'Connor NTJ, Wainscoat JS, Weatherall DJ, Gatter KC, Feller AC, Isaacson P, Jones D, Lennert K, Pallesen G, Ramsey A, Stein H, Wright DH, Mason DY: Rearrangement of the T-cell-receptor β-chain gene in the diagnosis of lymphoproliferative disorders. Lancet 1:1295–1296, 1983
Rambaldi A, Pelicci PG, Allavena P, Knowles DM, Rossini S, Bassan R, Barbui T, Dalla-Favera R, Mantovani A: T cell receptor β-chain gene rearrangements in lymphoproliferative disorder of large granular lymphocyte/natural killer cells. J Exp Med 162:2156–2162, 1985
van der Griend RJ, Bolhuis RLH: In vitro expansion and analysis of cloned cytotoxic T cells derived from patients with chronic T γ lymphoproliferative disorders. Blood 65:1002–1009, 1985
Uchiyama T, Broder S, Waldmann TA: A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac+ cells. J Immunol 126:1393–1397, 1981
Corte G, Damiani G, Calabi F, Fabbi M, Bargellesi A: Analysis of HLA polymorphism by two dimensional peptide mapping. Proc Natl Acad Sci USA 78:534–538, 1981
Ferrarini M, Cadoni A, Franzi AT, Ghigliotti C, Leprini A, Zicca A, Grossi CE: Ultrastructure and cytochemistry of human peripheral blood lymphocytes. Similarities between the cells of the third population and TG lymphocytes. Eur J Immunol 10:562–570, 1980
Itoh K, Tilden AB, Kumagai K, Balch CM: Leu 11+ lymphocytes with natural killer (NK) activity are precursors of recombinant interleukin 2 (rIL2)-induced activated killer (AK) cells. J Immunol 134:802–807, 1984
Standing Committee on Human Cytogenetic Nomenclature: An international system for human cytogenetic nomenclature. Cytogenet Cell Genet 21:309–404, 1978
Ferrarini M, Romagnani S, Montesoro E, Zicca A, Del Prete GF, Nocera A, Maggi E, Leprini A, Grossi CE: A lymphoproliferative disorder of the large granular lymphocytes with a natural killer activity. J Clin Immunol 3:30–39, 1983
Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA: Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med 155:1823–1841, 1982
Rosenberg S: Lymphokine-activated killer cells: A new approach to immunotherapy of cancer. J Natl Cancer Inst 75:595–603, 1985
Trinchieri G, Kobayashi M, Clark SC, Seehra J, London L, Perussia B: Response of resting human peripheral blood natural killer cells to interleukin 2. J Exp Med 160:1147–1169, 1984
Fu SM, Chiorazzi N, Kunkel HG: Differentiation capacity and other properties of the leukemic cells of chronic lymphocytic leukemia. Immunol Rev 48:23–44, 1979
Rubartelli AI, Sitia R, Grossi CE, Ferrarini M: Maturation of chronic lymphocytic leukemia B cells: Correlation between the capacity of responding to T cell factors in vitro and the stage of maturation reached in vivo. Clin Immunol Immunopathol 34:296–303, 1985
Pistoia V, Prasthofer EF, Tilden AB, Ferrarini M, Grossi CE, Barton JC, Zuckerman KS: Interleukin (IL)-2 and gamma interferon (γ-IFN) production by cell lines from patients with granular lymphocyte (GL) expansions or leukemias. Blood 66 (Suppl):101a, 1985
Third International Workshop on Chromosomes in Leukemia, 1980: Chromosomal abnormalities in acute lymphoblastic leukemia: Structural and numerical changes in 234 cases. Cancer Genet Cytogenet 4:101–110, 1981
Loughran TP, Kadin ME, Starkebaum G, Abkowitz JL, Clark EA, Disteche C, Lum LG, Slichter SJ: Leukemia of large granular lymphocytes: Association with clonal chromosomal abnormalities and autoimmune neutropenia, thrombocytopenia and hemolytic anemia. Ann Intern Med 102:169–175, 1985
McKenna RW, Arthur DC, Gajl-Peczalska KJ, Flynn P, Brunning RD: Granulated T cell lymphocytosis with neutropenia: Malignant or benign chronic lymphoproliferative disorder? Blood 66:259–266, 1985
Landay A, Gebel H, Prasthofer E, Zarcone D, Naujokas M, Downing J, Grossi CE: Gene probe analysis of a unique natural killer lymphoproliferative disorder. Blood 66 (Suppl):177a, 1985
Ford RJ, Yoshimura L, Morgan J, Quesada J, Montagna R, Maizel A: Growth factor-mediated tumor cell proliferation in hairy cell leukemia. J Exp Med 162:1093–1098, 1985
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pistoia, V., Carroll, A.J., Prasthofer, E.F. et al. Establishment of Tac-negative, interleukin-2-dependent cytotoxic cell lines from large granular lymphocytes (LGL) of patients with expanded LGL populations. J Clin Immunol 6, 457–466 (1986). https://doi.org/10.1007/BF00915251
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00915251