Conclusions
-
1.
The limits of thermal stability of preparations containing up to 75% MgO2 were determined by the method of differential thermal analysis.
-
2.
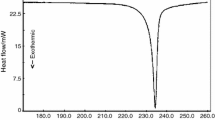
It was shown by an interpretation of the thermograms that these preparations release the bulk of the water of the adsorbed mother liquor at 110° and are entirely dehydrated at 260°. In this case there is a partial decomposition of MgO2 with evolution of oxygen and the formation of Mg(OH)2.
-
3.
The mixture of MgO2 and Mg(OH)2 formed decomposes exothermic ally within the range 360–375°, liberating oxygen and forming MgO. The following reactions are responsible for the exothermicity of the effect on the heating curves of magnesium peroxide preparations: MgO2+2H2O→Mg(OH)2+H2O2, H2O2→H2O+1/2 O2.
Similar content being viewed by others
Literature cited
I. I. Vol'nov and E. I. Latysheva, Zh. Neorgan. Khimii,12, 2836 (1966).
N. S. Kurnakov and V. V. Chernykh, Izv. In-ta Fiz.-Khim. Analiza, 485 (1926).
A. S. Suleimanov, Dissertation [in Russian], Kazan' (1952).
M. Blumenthal, Roczn. Chem.,13, 5 (1933).
M. Centnerzswer and M. Blumenthal, Bull. Int. Acad. Pol. Sci., Series A, 543 (1934/1935).
P. Allamagny, Rev. Chim. Min.,2, 648 (1965).
J. P. Coughlin, Bull. 542, Bureau of Mines, Washington (1954).
I. I. Vol'nov, Zh. Neorgan. Khimii,3, 539 (1958).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 1, pp. 13–18, January, 1970.
Rights and permissions
About this article
Cite this article
Vol'nov, I.I., Latysheva, E.I. Thermal stability of magnesium peroxide. Russ Chem Bull 19, 11–15 (1970). https://doi.org/10.1007/BF00913914
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00913914