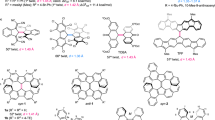
Abstract
The absolute configuration of 2.2′-spirobiindane-1.1′-dione (2) was established as (+)-(2S) by correlation with a centrochiral key intermediate, namely by cyclization of (+)-(2R)-2-benzyl-2-methoxycarbonyl-1-indanone (9) withPPA.9 was obtained by oxidation of either methyl (+)-cis- ortrans-1-hydroxy-2-benzyl-indane-2-carboxylate (8 a, 8 b), whose absolute configurations were determined as (+)-(1S, 2R) and (1R, 2R), resp., byHoreau's method and whose optical purities by the NMR-method employing the esters with α-methoxy-α-trifluoromethylphenyl-acetic acid. The active methyl esters8 were prepared starting from the corresponding racemic ethyl ketocarboxylate4, which on reduction afforded the stereoisomeric ethylcis- andtrans-1-hydroxyindane-2-benzyl-2-carboxylates (5 a, 5 b); their relative configurations were determined by NMR. The corresponding acids7 were resolvedvia the cinchonidine salts and obtained in high optical purity (100% fortrans, 80% forcis).
Levorotatory2 was also prepared by resolution of the bis-aminoxyacetic acid derivative (via its bis-cinchonidine salt) and subsequent cleavage. [a] maxD of2 was determined as ±240° (benzene) by means of a chiral shift reagent. The racemization of2 withPPA under the conditions of the asymmetric synthesis was studied.
Similar content being viewed by others
Literatur
Mitt.:H. Falk, W. Fröstl undK. Schlögl, Mh. Chem.102, 1270 (1971).
D. J. Caldwell andH. Eyring, The Theory of Optical Activity. New York: Wiley-Interscience. 1971.
Vgl. z. B.:O. Aschan, Ber. dtsch. chem. Ges.35, 3389 (1902);H. Leuchs, Ber. dtsch. chem. Ges.46, 2435 (1913);W. H. Mills undC. K. Nodder, J. Chem. Soc.117, 1407 (1920).
G. Krow undR. H. Hill, Chem. Commun.1968, 430;H. Gerlach, Helv. Chim. Acta51, 1587 (1968).
G. Haas undV. Prelog, Helv. Chim. Acta52, 1202 (1969);G. Haas, P. D. Hulbert, W. Klyne, V. Prelog undG. Snatzke, Helv. Chim. Acta54, 491 (1971).
S. Hagishita, K. Kuriyama, M. Hayashi, Y. Nakano, K. Shingu undM. Nakagawa, Bull. Chem. Soc. Japan44, 496, 617, 2177 (1971).
J. H. Brewster undR. T. Prudence, J. Amer. Chem.95, 1217 (1973);R. K. Hill undD. A. Cullison, J. Amer. Chem. Soc.95, 1229 (1973).
H. Leuchs undJ. Wutke, Ber. dtsch. chem. Ges.46, 2420 (1913).
C. Rappe undH. Bergander, Acta Chem. Scand.23, 214 (1969).
H. Falk undW. Fröstl, Mh. Chem.102, 1259 (1971).
Vorläufige Mitt.:H. Falk, W. Fröstl undK. Schlögl, Tetrahedron Letters1974, 217.
Vgl. z. B.:R. P. Linstead undA. F. Millidge, J. Chem. Soc.1933, 1065.
F. Elsinger, J. Schreiber undA. Eschenmoser, Helv. Chim. Acta,43, 113 (1960).
M. Raban undK. Mislow, in: Topics in Stereochemistry, Bd. II, S. 199. New York: Interscience. 1967.
J. A. Dale, D. L. Dull, andH. S. Mosher, J. Org. Chem.34, 2543 (1969).
K. E. Pfitzner undJ. G. Moffatt, J. Amer. Chem. Soc.85, 3027 (1963);87, 5661 und 5670 (1965).
Vgl. z. B.:A. Horeau, Tetrahedron Letters1961, 506;1962, 965;K. Schlögl, in: Methodicum Chimicum, Bd. I/1, S. 235. Stuttgart: Thieme. 1973.
M. J. Luche, A. Marquet undG. Snatzke, Tetrahedron28, 1677 (1972).
R. A. Barnes undB. D. Breitchman, J. Amer. Chem. Soc.76, 5430 (1954).
W. Baker, J. F. W. McOmie, S. D. Parfitt undD. A. M. Watkins, J. Chem. Soc.1957, 4026.
S. F. Torf, N. Y. Khormov-Borisov, E. N. Gur'yanova undI. P. Goldshtein, Zh. Org. Khim.3, 1506 (1967); Chem. Abstr.68, 2723 (1967).
R. Weißgerber, Ber. dtsch. chem. Ges.44, 1436 (1911).
H. Gerlach undW. MÜller, Angew. Chem.84, 1110 (1972); Internat. Ed. 1030.
E. Langer undH. Lehner, Tetrahedron29, 375 (1973). Für eine neuere Synthese von racem.2 vgl.E. Dynesen, Acta chem. Scand.26, 850 (1972).
H. Leuchs undD. Radulescu, Ber. dtsch. chem. Ges.45, 189 (1912).
J. G. S. C. Buchanan undP. D. Woodgate, Quart. Rev. Chem. Soc.23, 522 (1969);S. A. Galton, M. Kalafer undF. M. Beringer, J. Org. Chem.35, 1 (1970).
R. S. Cahn, C. K. Ingold undV. Prelog, Angew. Chem.78, 413 (1966); Intern. Ed.5, 385 (1966).
R. B. Woodward, T. P. Kohman undG. C. Harris, J. Amer. Chem. Soc.63, 120 (1941).
H. Kaehler, F. Nerdel, G. Engemann undK. Schwerin, Ann. Chem.757, 15 (1972).
A. J. McBay, G. C. Jenkins undJ. B. Data, J. Amer. Pharm. Assoc.39, 138 (1950); C. A.44, 4870 d (1950).
A. Courtot, Ann. Chim. (France)4, 58 (1915).
K. Wiechert, Z. anorg. Chem.261, 310 (1950).
H. Leuchs undL. Lock, Ber. dtsch. chem. Ges.48, 1432 (1915).
A. Latif undG. Soliman, J. Chem. Soc.1944, 53.
E. Borek undH. T. Clarke, J. Amer. Chem. Soc.58, 2020 (1936).
P. Block undM. S. Newmann, Org. Synth.48, 120 (1968) (Synthese der entsprechendenPropionsäure).
Author information
Authors and Affiliations
Additional information
Mit 4 Abbildungen
Rights and permissions
About this article
Cite this article
Falk, H., Fröstl, W. & Schlögl, K. Darstellung, absolute Konfiguration und optische Reinheit von 2,2′-Spiro-biindan-1,1′-dion. Monatshefte für Chemie 105, 574–597 (1974). https://doi.org/10.1007/BF00912610
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00912610