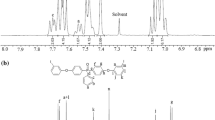
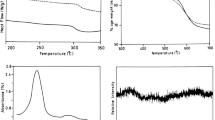
Conclusions
-
1.
Polyamides were prepared from 3,3′-(phenylphosphinylidene)dipropionic acid with 1,6-hexanediamine, 1,8-octanediamine, m- and p-phenylenediamines,and toluenediamine.
-
2.
The polyamides derived from aromatic diamines have higher melting points than polyamides from aliphatic diamines.
-
3.
Polyamides were prepared by the polycondensation of 3,3'-(phenylphosphinylidene)bispropylamine with aliphatic dicarboxylic acids and with dimethyl terephthalate.
-
4.
All the polyamides synthesized have the property of self-extingushing on removal from a flame.
Similar content being viewed by others
Literature cited
V. V. Korshak, J. Polymer Sci.,31, 319 (1958).
T. M. Frunze, V. V. Korshak, V. V. Kurashev, G. S. Kolesnikov, and B. A. Zhubanov, Izv. AN SSSR, Otd. khim. n.,1958, 703.
T. M. Frunze, V. V. Korshak, and V. V. Kurashev, Vysokomolekul. soed.,1, 670 (1959).
K. A. Petrov and V. A. Parshina, Khim. nauka i prom.,5, 686 (1959).
K. A. Petrov and V. A. Parshina, Zh. obshch. khimii,30, 1342 (1960).
M. H. Bride, W. A. Cummings, and W. Pickles, J. Appl. Chem.,11, 352 (1961).
V. V. Korshak, T. M. Frunze, and V. V. Kurashev, Vysokomolekul. soed.,2, 633 (1960).
T. Ya. Medved', T. M. Frunze, Hu Ching-mei, V. V. Kurashev, V. V. Korshak, and M. I. Kabachnik, Vysokomolekul. soed.,5, 1309 (1963).
B. A. Arbuzov, G. M. Vinokurova, and I. A. Perfil'eva, DAN SSSR,127, 1217 (1959).
B. A. Arbuzov, G. M. Vinokurova, and I. A. Aleksandrova, Izv. AN SSSR, Otd. khim. n.,1962, 290.
B. A. Arbuzov and G. M. Vinokurova, Izv. AN SSSR, Otd. khim. n.,1963, 502.
I. Pellon and W. G. Carpenter, J. Polymer Sci., Part A1, 863 (1963).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 2, pp. 362–267, February, 1967.
It is our pleasant duty to express our sincere thanks to B. A. Arbuzov for constant interest in this work.
Rights and permissions
About this article
Cite this article
Vinokurova, G.M., Aleksandrova, I.A. Phosphorus-containing polymers. Russ Chem Bull 16, 343–346 (1967). https://doi.org/10.1007/BF00912441
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00912441