Abstract
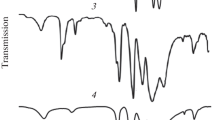
Zinc phosphites ZnPHO3·2.5 H2O, Zn2H2P3H3O9·H2O, Zn3H4P5H5O15·1.5 H2O, ZnH2H2P2H2O6 have been studied at higher temperatures and by X-rays and molecular spectroscopy. Hydrates ZnPHO3·2.5 H2O and Zn2H2P3H3O9·H2O, when heated, yield an anhydrous salt. Thermal decomposition of dihydrogen triorthophosphite and tetrahydrogen pentaorthophosphite leads, before oxidation of the anion, to a mixture of zinc phosphite ZnPHO3 and dihydrogen diorthophosphite ZnH2P2H2O6 and then after loss of water of constitution dihydrogen diorthophosphite converts to zinc diphosphite ZnP2H2O5. The results of the thermal decomposition study were confirmed by X-ray investigation. Anhydrous zinc dihydrogen triorthophosphite Zn2H2P3H3O9 and zinc diphosphite ZnP2H2O5 were hitherto unknown. Infrared spectra confirmed the existence of hydrogen bonding in all the phosphites studied and in the case of zinc phosphite ZnPHO3·2.5 H2O exhibited a symmetry decrease of the anion PHO3 2− from the point group C3v to Cs. In the crystal lattice of ZnPHO3·2.5 H2O hydrogen bonding by water molecules participates, with polyorthophosphites hydrogen bonding shares in the production of anions and in the case of their hydrates there is in addition hydrogen bonding by water molecules.
Similar content being viewed by others
Literatur
M. Ebert undM. Pelikánová, Collect. Czechoslov. Chem. Commun.37, 3672 (1972).
C. F. Rammelsberg, Poggendorffs Ann.132, 481 (1867).
J. H. Hanawalt, H. W. Rinn undL. K. Frevel, Ind. Engng. Chem., Anal. Ed.10, 457 (1938).
A. Jílek undJ. Kota, Vážková analysa a elektroanalysa. Praha: 1951.
R. Přibil undJ. Jelínková, Chem. listy46, 400 (1952).
J. Körbl, R. Přibil undA. Emer, Chem. Listy50, 1440 (1956).
J. Nassler, Collect. Czechoslov. Chem. Commun.28, 3424 (1953).
K. H. Russell, The Vibrational Spectra of Phosphorous Acid and Its Salts. Thesis, Washington State University, 1964.
M. Ebert undJ. Eysseltová, Mh. Chem.100, 553 (1969).
M. Ebert undJ. Eysseltová, Mh. Chem.103, 188 (1972).
N. D. Sokolov, Usp. fiz. nauk57, 205 (1955).
K. Nakamoto, M. Margoshes undR. E. Rundle, J. Amer. Chem. Soc.77, 6480 (1955).
H. Ratajczak undW. J. Orville-Thomas, J. Mol. Structure1, 449 (1968).
J. J. Efimov undJ. I. Naberuchin, J. strukt. chim.12, 591 (1971).
S. N. Andreev undT. G. Baličeva, Dokl. akad. nauk SSSR148, 86 (1963).
A. V. Karjakin undG. A. Muradova, J. fiz. chim.42, 2735 (1968).
R. E. Hester undR. A. Plane, Inorg. Chem.3, 768 (1964).
J. R. Ferraro undA. Walker, J. Chem. Phys.42, 1278 (1965).
M. Ebert, J. Eysseltová undA. Rottová, Collect. Czechoslov. Chem. Commun.35, 1824 (1970).
M. Ebert, Chemiker-Ztg.44, 839 (1970).
B. Barnoyer, G. Brun undM. Maurin, Rév. Chim. Min.7, 941 (1970).
H. Ratajczak, J. Mol. Struct.3, 27 (1969).
E. R. Lippincott undR. Schroeder, J. Chem. Phys.23, 1099 (1955).
C. Reid, J. Chem. Phys.30, 182 (1959).
E. E. Berry undC. B. Baddiel, Spectrochim. Acta23 A, 1781 (1967).
Author information
Authors and Affiliations
Additional information
Mit 3 Abbildungen
Rights and permissions
About this article
Cite this article
Ebert, M., Pelikánová, M. Über einige Eigenschaften von Zinkphosphiten mit Berücksichtigung ihrer Wasserstoffbrückenbindung. Monatshefte für Chemie 105, 11–18 (1974). https://doi.org/10.1007/BF00911282
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00911282