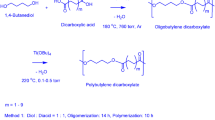
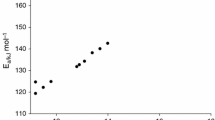
Summary
-
1.
The emanation method was used to study the processes occurring during the heating of barium phosphotungstate.
-
2.
The emanation of the salt at room temperature is higher, the higher its water content.
-
3.
Regardless of the initial water content of the original hydrates, the changes occurring during heating above 60‡ are the same in character.
-
4.
Over the temperature range from 100 to 200‡, where a large amount of water is lost, dehydration is not accompanied by a substantial change in emanation.
-
5.
Practically complete dehydration (350‡) is not connected with the decomposition of the heteropolyanion, which is quite stable and begins to decompose at 580‡.
-
6.
Barium phosphate and tungsten trioxide were found among the thermal decomposition products of barium phosphotungstate.
Similar content being viewed by others
Literature cited
L. S. Kolovrat-Chervinskii, Work of the Radium Expedition of the Russian Academy of Sciences, No. 9 (1918); Le Radium4, 317 (1907);6, 321 (1909).
K. Zimmens, Z. phys. Chem. A.191, 1, 95 (1942); S. Flugge and K. Zimmens, Z. phys. Chem. B.42, 179 (1939).
K. Zimmens, Z. phys. Chem. B.37, 231 (1937);192, I (1943).
G. M. Zhabrova, M. D. Sinitsyna, and S. Z. Roginskii, Dokl. AN SSSR117, 255 (1957).
K. B. Zaborenko, A. M. Babeshkin, and V. A. Georgieva, Radiokhimiya, No. 3, 336 (1959).
M. Spenger, J. prakt. Chem.22, 428 (1880).
W. Gibbs, Proc. Amer. Acad.16, 122 (1881).
M. N. Sobolev, Zh. russk. fiz.-khim. obsh.28, 187 (1896).
A. Rosenheim, and J. Jaenicke, Z. anorgan. Chem.101, 854 (1907).
A. Ferrari, L. Cavalka, and M. Nardelli, Gazz. chem. ital.78, 551 (1948).
E. A. Nikitina and N. E. Kulakova, Z. neorgan. khimii4, 564 (1959).
G. Brauer, Textbook of Preparative Inorganic Chemistry [Russian translation], IL, 1956.
E. A. Nikitina, Z. obshch. khimii7, 889, 2609 (1937); E. A. Nikitina and O. N. Sokolova, Z. obshch. khimii23, 1437 (1953).
A. V. Rakovskii and E. A. Nikitina, Z. obshch. khimii1, 240 (1931).
A. M. Babeshkin, V. I. Baranov, and K. B. Zaborenko, Zavodsk. laboratoriya, No. 8, 996 (1958).
B. Sabortschev, Z. phys. Chem. A.176, 295 (1931).
E. Ya. Rode, Z. neorgan. khimii3, 2717 (1958).
A. A. Babad-Zakhryapin, Z. neorgan. khimii3, 2313 (1958).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Spitsyn, V.I., Zaborenko, K.B., Radicheva, M.A. et al. Application of the emanation method to the study of conversions of heteropoly compounds. Russ Chem Bull 10, 3–8 (1961). https://doi.org/10.1007/BF00909394
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00909394