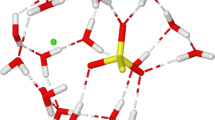
Abstract
CNDO/2-MO-SCF calculations have been performed for the system ClO4 −−HCOOH, which indicated small solvation energy and very small changes in molecular geometries as a result of solvation, but higher solvation numbers for ClO4 −, compared to monoatomic anions. These results have been examined by means of1H-NMR investigations of solutions of NaCl, NaClO4, NaBr and Na2SO4 in HCOOH. The experimental data agree with the conclusions drawn from quantum chemical results. The semiempirical SCF calculations proved as valuable stimulation and supplement for the experimental working.
Similar content being viewed by others
Literatur
B. M. Rode, Z. anorg. allgem. Chem.399, 239 (1973).
B. M. Rode, Chem. Phys. Letters20, 366 (1973).
B. M. Rode, J. Chem. Soc., Faraday Trans. II1973, 1439.
J. A. Pople, D. P. Santry undG. A. Segal, J. Chem. Phys.43, 129 (1965).
J. A. Pople undG. A. Segal, J. Chem. Phys.43, 136 (1965);44, 3289 (1966).
P. Schuster, Internat. J. Quantum Chem.3, 851 (1969).
B. M. Rode, A. Engelbrecht undW. Jakubetz, Chem. Phys. Letters18, 285 (1973).
P. Schuster undTh. Funck, Chem. Phys. Letters8, 587 (1968).
Quantum Chemistry Program Exchange. Bloomington, Indiana, USA.
G. P. Kotlyarova undE. F. Ivanova, Russ. J. Phys. Chem.40, 537 (1966).
G. P. Kotlyarova undE. F. Ivanova, Russ. J. Phys. Chem.38, 221 (1964).
P. Russegger undP. Schuster, Chem. Phys. Letters19, 254 (1973).
Houben-Weyl, Methoden der org. Chemie, Bd. I/2, S. 321. Stuttgart: G. Thieme. 1959.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rode, B.M. Die Wechselwirkung des ClO4-Ions mit HCOOH als Solvens. Monatshefte für Chemie 105, 308–313 (1974). https://doi.org/10.1007/BF00907377
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00907377