Summary
-
1.
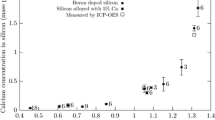
Calcium sulfide forms high-temperature solid solutions in mixed calcium and magnesium silicates which decompose with reduction in temperature. The solubility of calcium sulfide in the studied silicates has a restricted character.
-
2.
High-temperature solid solutions based on pseudowollastonite in the CaO-MgO-SiO2-CaS system contain not only CaS but also MgSiO3 with the probable formula of solid solutions(Ca, Mg)Si(O, S)3.
-
3.
A connection has been observed between the change in phase composition and viscosity of the melts.
Similar content being viewed by others
Literature cited
P. Lebedev, Izv. S.-Pb. Politekhn. in-ta13, 613 (1910).
A. Voloskov, Izv. S.-Pb. Politekhn. in-ta15, 42 (1911).
J. H. L. Vogt, Die Sulfid. Silikat Schmelzlosungen, Kristianta, 1919.
O. Glaser, Cbl. Mining, Abt. A, 81 (1926).
R. S. McCaffery and J. F. Oesterle, Trans. Amer. Inst. Mining a. Metall. Enging.69, 606–634 (1925).
B. P. Selivanov, A. S. Ginzberg, and S. I. Nikol'skii, Soobshch. in-ta metallov, No. 8, 25 (1931).
B. P. Selivanov, A. S. Ginzberg, and S. I. Nikol'skii, Soobshch. in-ta metallov, No. 11, 111 (1933).
A. I. Tsvetkov and I. V. Borisevich, Jubilee Collection in Honor of Academician D. S. Belyankin's 70th Birthday [in Russian], 1946, p. 498.
Ya. I. Ol'shanskii, Transactions of the Conference on Experimental Petrography [in Russian], Vol. 1, (1951).
Ya. I. Ol'shanskii, Tr. In-ta geol. nauk AN SSSR121, 12(1950).
A. S. Panov and A. V. Rudneva, Izv. vysshikh uchebnykh zavedenii, Chernaya metallurgiya, 1961, p. 11.
A. N. Winchell and G. Winchell, Optical Mineralogy [Russian translation], IL, M., (1953).
E. F. Osborn, R. C. De Vries, K. H. Gee, and H. M. Kraner, J. of Metals, June, 1954, pp. 35–39; Problemy sovremennoi metallurgii, 1955, p. 2, 23–43.
K. P. Abraham and F. D. Richardson, Metallurg. Soc. Conf. 1961, pp. 263–274.
A. S. Panov, I. S. Kulikov, and L. M. Tsylev, Izv. AN SSSR. Otd. tekhn. n., Metallurgiya i toplivo3, 25–30 (1961).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rudneva, A.V., Panov, A.S. The effect of calcium sulfide on the phase composition of slags in the system CaO-MgO-SiO2 . Russ Chem Bull 11, 510–513 (1962). https://doi.org/10.1007/BF00904743
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00904743