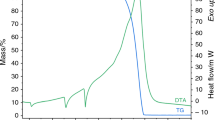
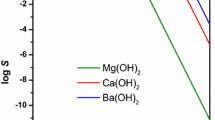
Conclusions
-
1.
The radiolysis of aqueous solutions of potassium hydroxylaminedisulfonate under various conditions of irradiation (concentration, pH, introduction of additives, etc.) was studied by the EPR method.
-
2.
The results obtained indicate a radiolytic oxidation of HON(SO3K)2 by OH radicals.
-
3.
The high conversion yields of HON(SO3K)2 dissolved in water are treated on the basis of the concept of the participation of the conversion products of excited water molecules in the reactions.
-
4.
The system HON(SO2K)3 + H2O can be recommended as an indicator system for determining the rate constant of the reaction of the oxidative component of the radiolysis of water with dissolved substances.
Similar content being viewed by others
Literature cited
V. A. Sharpatyi, Uspekhi Khirnii,30, 645 (1961).
M. A. Proskurnin, V. D. Orekhov, and E. V. Barelko, Uspekhi Khimii,24, 584 (1955).
V. D. Orekhov and A. A. Zansokhova, Problems of Physical Chemistry [in Russian],2, Goskhimizdat (1959), p. 194.
V. A. Sharpatyi, V. D. Orekhov, and M. A. Proskurnin, Dokl. Akad. Nauk SSSR,122, 852 (1958).
V. A. Sharpatyi and M. A. Proskurnin, Transactions of the Second All-Union Conference on Radiation Chemistry [in Russian], Izd-vo AN SSSR (1962), p. 122.
V. A. Sharpatyi and Yu. N. Molin, Transactions of the Second All-Union Conference on Radiation Chemistry [in Russian], Izd-vo AN SSSR (1962), p. 141.
V. A. Sharpaty, Radiation Chemistry, Proc. of the I Tihany Symposium on Radiation Chemistry 1962, Budapest (1964), p. 463.
V. A. Sharpaty, D. M. Margolin, N. V. Zakatova, and K. G. Yanova, Radiation Chemistry, Proc. of the II Tihany Symposium on Radiation Chemistry 1966, Budapest (1967), p. 187.
G. V. Buston and F. S. Dainton, Proc. Royal Soc.,A287, No. 1410, 427 (1965).
S. Gordon, E. J. Hart, M. S. Matheson, J. Rabani, and J. K. Thomas, Disc. Faraday Soc.,36, 193 (1963).
N. S. Fel', V. I. Zolotarevskii, N. V. Zakatova, and V. A. Sharpatyi, Khim. Vys. Énerg.,2, 376 (1968).
V. A. Sharpatyi and Yu. N. Molin, Zh. Fiz. Khimii,35, 1465 (1961).
V. A. Sharpatyi, V. D. Orekhov, and M. A. Proskurnin, Collection: Effects of Ionizing Radiations on Inorganic and Organic Systems [in Russian], Izd-vo AN SSSR (1958), p. 43.
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 10, pp. 2197–2203, October, 1968.
Rights and permissions
About this article
Cite this article
Sharpatyi, V.A., Zakatova, N.V. Radiolysis of aqueous solutions of potassium hydroxylaminedisulfonate. Russ Chem Bull 17, 2082–2086 (1968). https://doi.org/10.1007/BF00904019
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00904019