Abstract
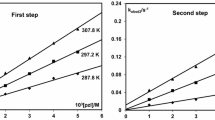
Polarographic study of Pd(II) reveals that it reduces quasireversibly at d.m.e. in 0.2M-pyridine+0.1N-HCl medium. Kinetic parameters of Pd(II) in various concentrations of α-resorcyclic acid were calculated usingGellings method and thus the effect of concentration of α-resorcyclic acid on the kinetics of the reduction of Pd(II) has been explained.DeFord/Hume graphical extrapolation method and the mathematical method ofMihailov have been employed for the evaluation of overall formation constants of the complexes formed with α-resorcyclic acid. The logarithmic values of overall formation constants β1, β2 and β3 obtained by the two methods are 7.47, 8.60, 9.66 (DeFord andHume Method) and 7.44, 8.64, 9.66 (Mihailov Method) at 298 K. Thermodynamic parameters of these complexes are reported.
Zusammenfassung
Pd(II) zeigt eine quasireversible Reduktion in 0,2M-Pyridin/0,1N-HCl. Kinetische Parameter wurden mittels der Methode vonGelling ermittelt und damit der Effekt verschiedener α-Resorcylsäurekonzentrationen erklärt. Bildungskonstanten der entsperechenden Komplexe wurden nachDeFord/Hume und nachMihailov ermittelt. Die logarithmischen Werte der Gesamtbildungskonstanten β1, β2 und β3 bei 298 K betragen nach derDe Ford/Hume-Methode 7,47, 8,60 und 9,66, nach derMihailov-Methode 7,44. 8,64 und 9,66. Die thermodynamischen Parameter der Komplexbildung werden angegeben.
Similar content being viewed by others
References
L. Meites, Principles of Polarography, new ed., Appendix. New York: Interscience Pub.
R. J. Magee andW. H. Douglas, J. Electrochem.9, 361 (1965).
D. D. DeFord andD. N. Hume, J. Amer. Chem. Soc.73, 5321 (1951).
M. H. Mihailov, J. Inorg. Nucl. Chem.36, 107, 114 (1974).
P. J. Gellings, El. Chem. Ber. Bunsenges. Phys. Chem.66, 477, 481, 799 (1962);67, 167 (1963).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gaur, J.N., Baghel, S.C. & Sharma, R.S. Electrode kinetics and thermodynamic study of complexes of palladium(II) at D.M.E.. Monatshefte für Chemie 112, 439–443 (1981). https://doi.org/10.1007/BF00901823
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00901823