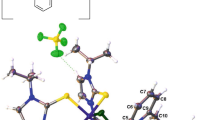
Abstract
The crystal structure of the paramagnetic bis(pyridine-2,6-dithiocarbomethylamide) nickel(II) nitrate (NiPDTA) is described: C18H22N6S4·(NO3)2·(H2O)1,5, monoclinic, C2/c,Z=4,a=14.705 (3) Å,b=23.254 (8) Å,c=8.383 (3) A, β=98.18 (2)°,d x=1.55 gcm−3,d m=1.53 gcm−3. The structure was solved withPatterson and differenceFourier techniques and refined to a residual ofR=0.053. The nickel is surrounded by a square bipyramidal coordination of four thioamide sulfur atoms and two pyridine nitrogen atoms. Vibrational and electronic band positions for this compound are discussed.
Zusammenfassung
Die Kristallstruktur des paramagnetischen bis(Pyridin-2,6-dithiocarbomethylamid) Nickel(II)-nitrats (NiPDTA) wurde bestimmt. C18H22N6S4Ni·(NO3)2·(H2O)1,5, monoklin, C2/c,Z=4,a=14,705 (3) Å,b=23,254 (8) Å,c=8,383 (3) A, β=98,18 (2)°,d x=1,55gcm−3,d m=1,53gcm−3. Das Phasenproblem wurde mittelsPatterson-und Differenz-Fourier-Synthese bestimmt und die Struktur bis zu einem kristallographischenR-Faktor vonR=0.053 verfeinert. Das Nickel-Atom ist von vier Thioamid-Schwefelatomen und zwei Pyridin-Stickstoffatomen in quadratisch-bipyramidaler Anordnung umgeben. Schwingungsspektren und Anregungsspektren des Komplexes werden diskutiert.
Similar content being viewed by others
References
Gagliardi, E., Popitsch, A., Mh. Chem.103, 1337, (1972).
Popitsch, A., to be published.
We are obliged to the authorities of the ETH-Zürich for giving us the opportunity to use the diffractometer. Our special thanks are due to Prof.J. D. Dunitz and Mr.P. Seiler.
All calculations were carried out with the XRAY computer program system (J. M. Stewart, The XRAY system-version of 1976. Tech. Rep. TR-466. Computer Science Center, Univ. of Maryland, College Park, Md., USA).
Popitsch, A., Nachbaur, E., Neißl, W., Fritzer, H. P., Mh. Chem.,111, 1321 (1980);Neißl, W., Fritzer, H. P., Proc. XXI. Cell. Spectr. Inter., Ref. No. 110310, Cambridge, England (1979).
Dobramysl, W., Fritzer, H. P., Inorg. Nucl. Chem. Lett.14, 269 (1978).
Popitsch, A., Gagliardi, E., Schurz, J., Kratky, C., Mh. Chem.112, 537 (1981)
Graw, H., Robinson, W. R., Walton, R. A., Inorg. Nucl. Chem. Lett.7, 695 (1971);Quaglieri, P., Loiseleur, H., Thomas, G.., Acta Cryst.B28, 2583 (1972).
Pignedoli, A., Peyeronel, G., Antolini, L., Acta Cryst.B30, 2181 (1974).
Nardelli, M., Gasparri, G. F., Battistini, G. G., Dominiano, P., Acta Cryst.20, 349 (1966).
König, E., Kremer, S., Ligand Field Energy Diagrams. New York: Plenum Press. 1977.
Lever, A. B. P., Coordin. Chem. Rev.3, 119 (1968).
Weiss, A., Witte, H., Magnetochemie. Verlag Chemie. 1972.
Clark, R. J. H., Williams, C. S., Inorg. Chem.4, 350 (1965);Takemeto, J. H., Inorg. Chem.12, 949 (1973);Schläpfer, C. W., Saito, Y., Nakamoto, K., Inorg. Chim. Acta6, 284 (1972).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kratky, C., Schurz, J., Gagliardi, E. et al. Crystal structure and spectra of the Ni(II) complex of pyridine-2,6-dithio-carbomethylamide. Monatshefte für Chemie 112, 721–730 (1981). https://doi.org/10.1007/BF00899775
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00899775