Summary
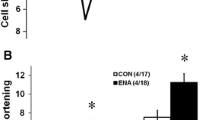
Angiotensin-converting enzyme (ACE) inhibitors protect the myocardium from experimental lethal ventricular arrhythmias induced by ischemia or reperfusion. Hypothetically, such arrhythmias may result from the calcium-dependent transient inward current Iti. It is already known that perindoprilat decreased the transient inward current in guinea-pig myocytes [1]. In the same preparation, however, angiotensin-IIdecreased the transient inward current, an effect opposite to that required to prove that the ACE inhibitor exerted its beneficial effects on Iti by lessening the action of angiotensin-II. We, therefore, selected another species, the rabbit, in which angiotensin-II was known to have a positive inotropic effect. Perindoprilat (1 µM but not 0.01 µM) decreased the transient inward current from −8.93±0.80 µA/cm2 to −5.33±0.74 µA/cm2 (p<0.05). Perindoprilat (1 µM) also protected from the effects of angiotensin-II (0.01 and 0.1 µM), which on its own increased the amplitude of the transient inward current. Based on our results, we conclude that perindoprilat (1 µM) prevents the effect of angiotensin-II in promoting the transient inward current in the rabbit. Hence our data support the hypothesis that the ACE inhibitor, perindoprilat, might in relatively high concentrations have an antiarrhythmic effect, at least in part through inhibition of angiotensin-II-evoked calcium-dependent Iti.
Similar content being viewed by others
References
Enous R, Coetzee WA, Opie LH. Effects of the ACE inhibitor, perindoprilat, and of angiotensin-II on the transient inward current of guinea pig ventricular myocytes.J Cardiovasc Pharmacol 1992;19:17–23.
Elfellah MS, Ogilvie RI. Effect of vasodilator drugs on coronary occlusion and reperfusion arrhythmias in anesthetized dogs.J Cardiovasc Pharmacol 1985;7:826–832.
Van Gilst WH, de Graeff PA, Kingma JH, Wesseling H, de Langen CDJ. Captopril reduces purine loss and reperfusion arrhythmias in the rat heart after coronary artery occlusion.Eur J Pharmacol 1984;100:113–117.
Van Gilst WH, de Graeff PA, Wesseling H, de Langen CDJ. Reduction of reperfusion arrhythmias in the ischemic isolated rat heart by angiotensin converting enzyme inhibitors: A comparison of captopril, enalapril and H0E 498.J Cardiovasc Pharmacol 1986;8:722–728.
De Graeff PA, van Gilst WK, Bel K, et al. Concentration-dependent protection by captoril against myocardial damage during ischemia and reperfusion in a closed chest pig model.J Cardiovasc Pharmacol 1987;9(Suppl 2):S37-S42.
Ribout C, Rochette L. Converting enzyme inhibitors (captopril, enalapril, perindoprilat) prevent early post-infarction ventricular fibrillation in the anaesthetized rat.Cardiovasc Drugs Ther 1987;1:51–55.
Rochette L, Ribout C, Belichard P, et al. Protective effect of angiotensin converting enzyme inhibitors (CEI): Captopril and perindoprilat on vulnerability to ventricular fibrillation during myocardial ischemia and reperfusion in rat.Clin Exp Theory Pract 1987;A9:365–368.
Muller CA, Opie LH, Peisach M, Pineda CA. Chronic oral pretreatment with angiotensin-converting enzyme inhibitor trandolapril decreases ventricular fibrillation and cardiac converting enzyme activity in a pig-model of acute ischemia and reperfusion.Eur Heart J 1994, in press.
Coetzee WA, Opie LH, Saman S. Proposed role of energy supply in the genesis of delayed afterdepolarizations—implications for ischemic or reperfusion arrhythmias.J Mol Cell Cardiol 1987;19(Suppl V):13–21.
Coetzee WA, Biermans G, Callewaert G, Vereecke J, Opie L, Carmeliet E. The effect of inhibition of mitochondrial energy metabolism on the transient inward current of isolated guinea-pig ventricular myocytes.J Mol Cell Cardiol 1988;20:181–185.
Opie LH, Coetzee WA, Dennis SC, Thandroyen FT. A potential role of calcium ions in early ischemic and reperfusion arrhythmias.Ann NY Acad Sci 1988;522:464–477.
Lubbe WF, Podzuweit T, Opie LH. Potential arrhythmogenic role of cyclic AMP and cytosolic calcium overload: Implications for antiarrhythmic effects of beta-blockers and proarrhythmic effects of phosphodiesterase inhibitors.J Am Coll Cardiol 1992;19:1622–1633.
Moravec C, Schuluchter MD, Paranandi L, et al. Inotropic effects of angiotensin-II on human cardiac muscle in vitro.Circulation 1990;82:1973–1984.
Berridge MJ. Inositol triphosphate and calcium mobilization.J Cardiovasc Pharmacol 1986;8(Supp 8):S85-S90.
Kentish JC, Barsotti RJ, Lea TJ, Mulligan IP, Patel JR, Ferenczi MA. Calcium release from cardiac sarcoplasmic reticulum induced by photorelease of calcium or Ins (1,4,5)P3.Am J Physiol 1990;258:H610-H615.
Kass RS, Blair ML. Effects of angiotensin-II on membrane current in cardiac Purkinje fibres.J Mol Cell Cardiol 1981;13:797–809.
Ohya Y, Sperelakis N. Involvement of a GTP-binding protein in stimulating action of angiotensin-II on calcium channels in vascular smooth muscle cells.Circ Res 1991;68:763–771.
Zhu Y, Nosek TM. Inositol trisphosphate enhances Ca2+ oscillations but not Ca2+-induced Ca2+ release from cardiac sarcoplasmic reticulum.Pflügers Arch 1991;418:1–6.
Heeg E, Meng K. Die Wirkung des Bradykinins, Angiotensins und Vasopressins auf Vorhof, Papilarmuskel und isoliert durchstromte Herzpraparate des Meerschweinchens.Naunyn Schmiedebergs Arch Pathol 1965;250:35–41.
Capogrossi MC, Kaku T, Filburn CR, et al. Phorbol ester and dioctanoylglycerol stimulate membrane association of protein kinase C and have a negative inotropic effect mediated by changes in cytosolic Ca2+ in adult rat cardiac myocytes.Circ Res 1990;66:1143–1155.
Opie LH.Angiotensin converting enzyme inhibitors: Scientific basis for clinical use. New York: Authors' Publishing House and Wiley-Liss, 1992:175.
Peach MJ, Cline WH, Davila D, Khairallah PA. Angiotensin-catecholamine interactions in the rabbit.Eur J Pharmacol 1970;11:286–292.
Ajayi AA, Campbell BC, Meredith PA, et al. The effect of captopril on the reflex control heart rate: Possible mechanisms.Br J Clin Pharmacol 1985;20:17–25.
Opie LH, Norris RM, Thomas M, Holland AJ, Owen P, van Noorden S. Failure of high concentrations of free fatty acids to provoke arrhythmias in experimental myocardial infarction.Lancet 1971;1:818–822.
Author information
Authors and Affiliations
Additional information
This article was evaluated by the San Francisco Editorial Office by two anonymous referees.
Rights and permissions
About this article
Cite this article
Enous, R., Opie, L.H. Effect of the angiotensin-converting enzyme inhibitor, perindoprilat, and of angiotensin-II on the transient inward current of rabbit ventricular myocytes. Cardiovasc Drug Ther 8, 647–651 (1994). https://doi.org/10.1007/BF00877418
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00877418