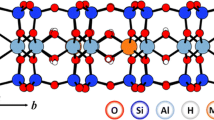
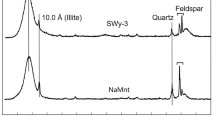
Abstract
The sorption mechanisms for trace metal ions on montmorillonite have been investigated. Complexation with surface hydroxyl groups located on the brocken edges of platelet particles is found to occur over a pH range similar to that observed on silica and other oxides, at comparable metal/site ratios. A second mechanism involving cation exchange on the negatively charge basal plane, which does not involve proton exchange in our experimental conditions, has been invoked to explain the low pH behavior. Consistent with this cation exchange mechanism, adsorption at low pH is strongly ionic strength dependant. A quantitative model which involves both mechanisms is presented and tested against both cation and proton adsorption data.
Similar content being viewed by others
References
Biederman, G., J. Bruno, D. Ferri, I. Grenthe, F. Salvatore and K. Spathiu, 1982. Mat. Res. Soc. Symp. Seri. 11:791.
Brown, C.S. and L.A. Thomas, 1960. The effect of impurities on the growth of synthetic quartz. J. Phys. Chem. Solids 13:337–342.
Bruggenwert, M. G. M. and A. Kamphorst, 1979. Survey of experimental information on cation exchange in soil systems. In: G. H. Bolt (ed.) Soil Chemistry B. Physicochemical Models. Elsevier, Amsterdam. pp. 141–203.
Charlet, L. and A. Manceau, 1993. Structure, formation and reactivity of hydrous oxide particles: In: J. Buffle and H. P. van Leeuwen (eds.), Environmental Particles II. Lewis Publ., pp. 117–164.
Dent, A.J., J.D.F. Ramsay and S.W. Swanton, 1992. An EXAFS study of uranyl ion in solution and sorbed onto silica and montmorillonite clay colloids. J. Colloid Interface Sci. 150:45–60.
Dougan, W.K. and A.L. Wilson, 1974. The absorptiometric determination of aluminum in water. A comparison of some chromogenic reagents and the development of an improved method. Analyst 99:413–430.
Fletcher, P. and G. Sposito, 1989. The chemical modelling of clay/electrolyte interactions for montmorillonite. Clay Minerals 24:375–391.
Goldberg, S. and R.A. Glaubig, 1986. Boron adsorption and silicon release by the clay minerals kaolinite, montmorillonite and illite. Soil Sci. Soc. Am. J. 50:1442–1448.
Hedlund, T., 1988. Studies of complexation and precipitation equilibria in some aqueous aluminum(III) systems. Ph.D. Thesis. Umeå University, Umeå, Sweden.
Hunter, R.J. and M. James, 1992. Charge reversal of kaolinite by hydrolyzable metal ions: an electroacoustic study. Clays Clay Minerals 40:644–649.
Iler, R.K., 1973. Effect of adsorbed alumina on the solubility of amorphous silica in water. J. Colloid Interface Sci. 43:399–408.
Lövgren, L., S. Sjörberg, and P.W. Schindler, 1990. Acid/base reactions and Al(III) complexation at the surface of goethite. Geochim. Cosmochim. Acta 54:1301–1306.
McBride, M.B., 1991. Processes of heavy and transition metal sorption by soil minerals. In: G. H. Bolt, F. DeBoot, M. H. B. Hayes and M. D. McBride (ed.) Interactions at the Soil Colloid-Soil Solution Interface. Kluwer Academic Press, The Netherlands. p. 149–175.
Motschi, H., 1987. Aspects of the molecular structure in surface complexes: Spectroscopic investigations. In: W. Stumm (ed.) Aquatic Surface Chemistry. John Wiley & Sons. p. 111–126.
Payne, T.E. and T.D. Waite, 1991. Surface complexation modelling of uranium sorption data obtained by isotopic exchange techniques. Radiochim. Acta 52/53:487–493.
Schindler, P.W., B. Fürst, R. Dick and P.U. Wolf, 1976. Ligand properties of surface silanol groups: I. Surface complex formation with Fe2+, Cu2+, Cd2+ and Pb2+. J. Colloid Interface Sci. 55: 469–475.
Schindler, P.W. and W. Stumm, 1987. The surface chemistry of oxides, hydroxides and oxide minerals. In: W. Stumm (ed.), Aquatic Surface Chemistry. John Wiley & Sons. p. 83–110.
Schindler, P.W., P. Lietchi, and J.C. Westall., 1987. Adsorption of copper, cadmium and lead from aqueous solution to the kaolinite/water interface. Netherlands J. Agri. Sci. 35:219–230.
Shaviv, A. and S. V. Mattigod, 1985. Cation exchange equilibria in soils expressed as cation-ligand complex formation. Soil Sci Soc. Am. J. 49:569–573.
Sposito, G., 1984. Surface Chemistry of Soils. Oxford University press, Oxford. 223 pp.
Sposito, G., K. M. Holtzclaw, C. T. Johnston and C. S. Le Vesque-Madore, 1981. Thermodynamics of sodium-copper exchange on Wyoming bentonite at 298 K. Soil Sci. Soc. Am. J. 45:1079–1084.
Stadler, M. and P. W. Schindler, 1993. Modeling of H+ and Cu2+ adsorption on calcium montmorillonite. Clays Clay Minerals 41:288–296.
Westall, J. C., 1982. FITEQL. A computer program for determination of chemical equilibrium constants from experimental data. Version 1.2, Report 82-01, Oregon State University, Corvallis, Oregon.
Wold, J. and W. F. Pickering, 1981. Influence of electrolytes on metal ion sorption by clays. Chem. Geol., 33:91–99.
Wolery, P.J., 1992. EQ 3/6: A software package for geochemical modeling of aqueous systems: package overview and installation guide. UCRL/MA/10662/Part I.
White, G. N. and L. W. Zelazny, 1988. Analysis and implications of the edge structure of dioctahedral phyllosilicates. Clays Clay Minerals 36:141–146.
Zhang, Y., L. Charlet and P. W. Schindler, 1992. Adsorption of protons, Fe(II) and Al(III) on lepidocrocite (γ — FeOOH). Colloids Surf. 62:259–268.
Zysset, M., 1992. Die protoneninduzierte Auflösung von K-Montmorillonit. Ph. D. Thesis. Bern University, Bern, Switzerland.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Charlet, L., Schindler, P.W., Spadini, L. et al. Cation adsorption on oxides and clays: The aluminum case. Aquatic Science 55, 291–303 (1993). https://doi.org/10.1007/BF00877274
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00877274