Summary
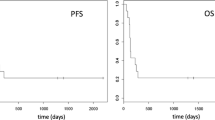
Fazarabine is a synthetic analog of cytosine arabinoside and 5-azacytidine that incorporates structural features of both compounds. Xenograft studies showed good activity against a variety of transplanted tumors. Initial studies in adults employed both a continuous infusion schedule and a daily bolus x 5 schedule. Myelotoxicity, especially neutropenia, was dose-limiting, with excessive myelotoxicity seen on the daily bolus x 5 at 72 mg/M2/day. Since short infusions may be administered in Ringer's lactate rather than either dimethylsulfoxide or dimethylacetamide required for continuous infusion, this study examined a daily x 5 schedule in children with refractory solid tumors. The initial dosage was 30 mg/M2/day, 80% of the maximum tolerated dosage in adults, with subsequent 30% dosage escalations. A total of 18 patients were enrolled, with a wide spectrum of pediatric solid tumors. Myelosuppression was the only significant toxicity, and was excessive at 78 mg/M2/day. Therefore, on this bolus regimen, 65 mg/M2/day for 5 days was the maximum tolerated dosage. One patient with medulloblastoma had stable disease for 65 days. No other responses were seen.
Similar content being viewed by others
References
Dalal M, Plowman J, Breitman TR, Schuller HM, delCampo AA, Vistica DT, Driscoll JS, Cooney DA, Johns DG: Arabinosyl-5-azacytosine: antitumor and cytotoxic properties. Cancer Res 46:831–838, 1986
Driscoll JS, Johns DG, Plowmnan J: Comparison of the activity of arabinosyl-5-azacytosine, arabinosyl cytosine, and 5-azacytidine against intracerebrally implanted L1210 leukemia. Invest New Drugs 3:331–334, 1985
Leiby JM, Malspeis L, Staubus AE, Kraut EH, Grever MR: Fazarabine (NSC 281272) is myelosuppressive at the initial dose level in a phase I study. Proc Am Soc Clin Oncol 7:62, 1988
Staubus AE, Malspeis L, Baleerzak SP, Leiby JM, Kraut EH, Grever MR: Clinical pharmacokinetics of fazarabine (NSC 281272). Proc Am Assoc Cancer Res 31:177, 1990
Kuebler JP, Metch B, Schuller DE, Keppen M, Hynes HE: Phase II study of fazarabine in advanced head and neck cancer: A Southwest Oncology Group study. Invest New Drugs 9:373–374, 1991
Heideman RL, Gillespie A, Ford H, Reaman GH, Balis FM, Tan C, Salo J, Ettinger LJ, Packer RJ, Poplack DG: Phase I trial and pharmacokinetic evaluation of fazarabine in children. Cancer Res 49:5213–5216, 1989
Fazarabine (Ara-AC, NSC 281272). National Cancer Institute Clinical Brochure. January, 1982
Bailey H, Tutschk D, Arzamanian RZ, Tombes MB, Alberti D, Bruggink J, Wilding G: Phase I clinical trial of fazarabine as a twenty-four-hour continuous infusion. Cancer Res 51:1105–1108, 1991
Surbone A, Ford H Jr., Kelley JA, Ben-Baruch N, Thomas RV, Fine R, Cowan KH: Phase I and pharmacokinetic study of arabinofuranosyl-5-azacytosine (Fazarabine, NSC 281272). Cancer Res 50:1220–1225, 1990
Walters RS, Theriault RL, Holmes FA, Hortobagy GN, Esparza L: Phase II trial of fazarabine (Ara-AC, arabinosyl5-azacytosine) in metastatic breast cancer. Invest New Drugs 10:43–44, 1992
Hubbard KP, Daugherty K, Ajani JA, Pazdur R, Levin B, Abbruzzese JL: Phase II trial of fazarabine in advanced colorectal carcinoma. Invest New Drugs 10:39–42, 1992
Johnston PG, Kleiner D, Cowan KH: Fazarabine: a report of response in a patient with multiply relapsed embryonal cell carcinoma. Am J Clin Oncol 16: 34–37, 1993
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bernstein, M.L., Whitehead, V.M., Grier, H. et al. A phase I trial of fazarabine in refractory pediatric solid tumors. Invest New Drugs 11, 309–312 (1993). https://doi.org/10.1007/BF00874429
Issue Date:
DOI: https://doi.org/10.1007/BF00874429