Conclusions
-
1.
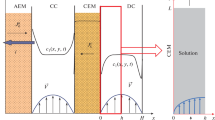
In the measurement of the transport numbers of ions, it is proposed that the solution as a whole be taken as the system of reading in solutions of electrolytes.
-
2.
The basic factors influencing the rate of motion of the ionic boundaries of electrolyte solutions under the application of electric current were discussed.
-
3.
The transport numbers of the ions H+, Li+, Na+, and K+ relative to the solutions were measured in the interval of concentrations of solutions of HCl, LiCl, NaCl, and KCl from 1N to saturated.
-
4.
Questions pertaining to the method of measuring the electroosmotic flow of solutions of electrolytes were discussed.
-
5.
The rates of electroosmotic flow of solutions of HCl, LiCl, NaCl, and KCl were measured in a broad range of variation of the concentrations. At concentrations of electrolyte solutions above 2N, and with specially selected sand fillers, the value of the electroosmotic flow of the solution is close to zero.
Similar content being viewed by others
Literature cited
W. Hittorf, Z. Phys. Chem.,39, 612 (1901).
G. Jones and B. C. Bradshaw, J. Amer. Chem. Soc.,54, 138 (1932).
D. A. MacInnes and I. A. Beattie, J. Amer. Chem. Soc.,42, 1117 (1920).
S. Lengyel, J. Giber, and J. Tomas, Acta chim. Acad. Scient, hung.,32, 429 (1962).
H. P. Cady and L. G. Longsworth, J. Amer. Chem. Soc.,51, 1656 (1929).
D. A. MacInnes, Longsworth, Chem. Revs,11, 171 (1932).
B. P. Konstantinov and V. P. Troshin, Zh. Prikl. Khimii,35, 2420 (1962).
E. A. Kaimakov and V. B. Fiks, Zh. Fiz. Khimii,35, 1777 (1961).
E. A. Kaimakov, Zh. Fiz. Khimii,36, 840 (1962).
A. A. Neyes and K. G. Falk, J. Amer. Chem. Soc.,33, 1436 (1911).
Collection: Electrokinetic Properties of Capillary Systems [in Russian], Izd. AN SSSR, Moscow (1956).
I. I. Zhukov, Colloid Chemistry [in Russian], Izd. LGU, Leningrad (1949).
B. P. Konstantinov and V. P. Troshin, Zh. Prikl. Khimii,36, 447 (1963).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 12, pp. 2104–2110, December, 1966.
Rights and permissions
About this article
Cite this article
Konstantinov, B.P., Troshin, V.P. Measurement of the transport numbers of ions relative to the solution in solutions of HCl, LiCl, NaCl, and KCl. Russ Chem Bull 15, 2039–2044 (1966). https://doi.org/10.1007/BF00867694
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00867694