Abstract
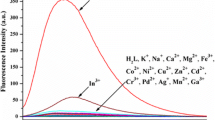
We have synthesizedN-methyl-9-anthrylhydroxamic acid, which is a fluorescent analogue ofN-methylbenzohydroxamic acid. Complexation with various di- and trivalent metal ions occurs (logK from 4 to 5) in water with resulting fluorescence quenching. Because the Fe(III) and Al(III) complexes substituted rather slowly, the addition of EDTA provides a temporal method for obtaining some selectivity in the chemosensor.
Similar content being viewed by others
References
G. Schwarzenbach and H. Flaschka (1969)Complexometric Titrations (transl. H. Irving), Methuen.
T. S. West (1969)Complexometry with EDTA and Related Reagents, BDH, Poole, UK.
E. Bishop (Ed.) (1972)Indicators, Pergamon.
G. G. Guilbault (1973)Practical Fluorescence, Marcel Dekker, New York, Chap. 6.
M. Huston, K. Haider, and A. W. Czarnik (1988)J. Am. Chem. Soc. 110, 4460–4462.
E. U. Akkaya, M. E. Huston, and A. W. Czarnik (1990)J. Am. Chem. Soc. 112, 3590–3593.
S. Mizukami and K. Nagata (1968)Coord. Chem. Rev. 3, 267–278.
S. Wathen (1992) Ph.D. thesis, Ohio State University, Columbus.
G. M. Steinberg and R. Swidler (1965)J. Org. Chem. 30, 2362–2365.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chae, MY., Czarnik, A.W. Fluorimetric metal ion sensing usingN-methyl-9-anthrylhydroxamic acid. J Fluoresc 2, 225–229 (1992). https://doi.org/10.1007/BF00865280
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00865280