Abstract
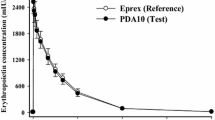
The single-dose pharmacokinetics of recombinant human erythropoietin (rHuEPO) given SC was investigated in 20 patients aged 7–20 years at different stages of chronic renal failure. In a pilot study we confirmed the lower bioavailability of the drug in 2 children when given SC compared with the IV route (24% and 43%, respectively). Following administration of 4,000 units/m2, rHuEPO SC effective serum erythropoietin concentrations increased from a mean baseline level (±SD) of 23±13 units/l to a mean peak concentration of 265±123 units/l, which was reached after 14.3±9.4 h, followed by a slow decline until baseline values were attained at 72 h. Mean residence time was 30±9 h and mean elimination half-time 14.3±7 h. The single-dose kinetics of SC rHuEPO in children with different degrees of renal failure are comparable to those in adult patients. Possibly, the higher efficacy of SC rHuEPO in patients with renal anaemia compared with IV rHuEPO is related to its prolonged action.
Similar content being viewed by others
References
Eschbach JW, Adamson JW (1985) Anemia of end-stage renal disease. Kidney Int 28:1–5
Schärer K, Müller-Wiefel DE (1987) Complications of renal failure. Haematological complications. In: Holliday MA, Barratt TM, Vernier RL (eds) Pediatric nephrology, 2nd edn. Williams and Wilkins, Baltimore, pp 880–887
Nissenson AR, Nimer SD, Wolcott DL (1991) Recombinant human erythropoietin and renal anemia: molecular biology, clinical efficacy, and nervous system effects. Ann Intern Med 114:402–416
Sinai-Trieman L, Salusky IB, Fine RN (1989) Use of subcutaneous recombinant human erythropoietin in children undergoing continuous cycling peritoneal dialysis. J Pediatr 114:550–554
Montini G, Zacchello G, Baraldi E, Zanconato S, Suppiej A, Fabris F, Andreetta B, Pavanello L, Zacchello F (1990) Benefits and risks of anemia correction with recombinant human erythropoietin in children maintained by hemodialysis. J Pediatr 117:556–560
Rigden SPA, Montini G, Morris M, Clark KGA, Haycock GB, Chantler C, Hill RC (1990) Recombinant human erythropoietin therapy in children maintained by haemodialysis. Pediatr Nephrol 4: 618–622
Offner G, Hoyer PF, Latta K, Winkler L, Brodehl J, Scigalla P (1990) One year's experience with recombinant erythropoietin in children undergoing continuous ambulatory or cycling peritoneal dialysis. Pediatr Nephrol 4:498–500
Scigalla P on behalf of the European Multicenter Study Group (1991) Effect of recombinant human erythropoietin treatment on renal anemia and body growth of children with end-stage renal disease. Contrib Nephrol 88:201–211
Warady BA, Sabarth RJ, Smith CA, Alon U, Hellerstein S (1991) Recombinant human erythropoietin therapy in pediatric patients receiving long-term peritoneal dialysis. Pediatr Nephrol 5:718–723
Schärer K, Müller-Wiefel DE, Braun A, Schaefer F, Böhler T. Erythropoietin therapy in children with renal anemia. In: Strauss J (ed) Renal disease dynamics. University of Miami Press, Coral Gables, Florida (in press)
Macdougall IC, Roberts DE, Naubert P, Dhermasena AD, Coles GA, Williams JD (1989) Pharmacokinetics of recombinant human erythropoietin in patients on continuous ambulatory peritoneal dialysis. Lancet I:425–427
Boelaert JR, Schurgers ML, Matthys EG, Belpaire FM, Daneels RF, De Cre MJ, Bogaert MG (1989) Comparative pharmacokinetics of recombinant erythropoietin administered by the intravenous, subcutaneous and intraperitoneal routes in continuous ambulatory peritoneal dialysis (CAPD) patients. Perit Dial Int 9:95–98
Kindler J, Eckardt KU, Ehmer B, Jandeleit K, Kurtz A, Schreiber A, Scigalla P, Sieberth HG (1989) Single-dose pharmacokinetics of recombinant human erythropoietin in patients with various degrees of renal failure. Nephrol Dial Transplant 4:345–349
Salmonson T (1990) Pharmacokinetic and pharmacodynamic studies on recombinant human erythropoietin. Scand J Urol Nephrol [Suppl] 129:1–66
Bommer J, Barth HJ, Zeier M, Mandelbaum A, Bommer G, Ritz E, Reichel H, Novack R (1991) Efficacy comparison of intravenous and subcutaneous recombinant human erythropoietin administration in hemodialysis patients. Contrib Nephrol 88:136–143
McMacon LP, Dawborn JK (1990) Experience with low dose intravenous and subcutaneous administration of recombinant human erythropoietin. Am J Nephrol 10:404–408
Egrie JC, Eschbach JW, McGuire T, Adamson JW (1988) Pharmacokinetics of recombinant human erythropoietin (rHuEPO) administered to haemodialysis patients (abstract). Kidney Int 33:262
Cotes PM, Pippard MJ, Reid CDL, Winearls CG, Oliver DO (1989) Characterization of the anemia of chronic renal failure and the mode of its correction by a preparation of human erythropoietin (rHuEPO): an investigation of the pharmacokinetics of intravenous erythropoietin and its effect on erythrokinetics. Q J Med 70:113–137
Faulds D, Sorkin EM (1989) Epoetin (recombinant human erythropoietin). A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in anaemia and the stimulation of erythropoiesis. Drugs 38:863–899
Eckardt KU, Kurtz A, Hirth P, Scigalla P, Wieczorek L, Bauer C (1988) Evaluation of the stability of human erythropoietin in samples for radioimmunoassay. Klin Wochenschr 66:241–245
Eckardt KU, Hartmann W, Vetter U, Pohlandt F, Burghardt R, Kurtz A (1990) Serum immunoreactive erythropoietin of children in health and disease. Eur J Pediatr 149:459–464
Rowland M, Tozer TN (1989) Clinical pharmacokinetics: concepts and applications, 2nd edn. Lea and Febiger, Philadelphia
Holford N (1988) MKMODEL. An extended least squares modelling program, 2nd edn. Biosoft, Cambridge, UK
Wallis WA, Roberts HV (1960) Statistics. A new approach. The Free Press, Glencoe, Illinois
Neumayer HH, Brockmöller J, Fritschka E, Roots I, Scigalla P, Wattenberg M (1989) Pharmacokinetics of recombinant human erythropoietin after SC administration and in long-term IV treatment in patients on maintenance hemodialysis. Contrib Nephrol 76: 131–142
Flaharty KK, Caro J, Erslev, Whalen JJ, Moris EM, Bjornsson TD, Vlasses PH (1990) Pharmacokinetic and erythropoietic response to human recombinant erythropoietin in healthy men. Clin Pharmacol Ther 47:557–564
Stockenhuber F, Loibl U, Gottsanner-Wolf M, Jahn CH, Manker W, Meisl TF, Balcke P (1991) Pharmacokinetics and dose response after intravenous and subcutaneous administration of recombinant erythropoietin in patients on regular hemodialysis treatment or continuous ambulatory peritoneal dialysis. Nephron 59:399–402
Evans JHC, Brocklebank JT, Bowner CJ, Ng PC (1991) Pharmacokinetics of recombinant human erythropoietin in children with renal failure. Nephrol Dial Transplant 6:709–714
Lui SE, Chung WWM, Leung CB, Chan K, Lai KN (1990) Pharmacokinetics and pharmacodynamics of subcutaneous and intraperitoneal administration of recombinant human erythropoietin in patients on continuous ambulatory peritoneal dialysis. Clin Nephrol 33:47–51
Wilton P, Widlund L, Guibaud O (1987) Bioequivalence of genotropin and somatonorm. Acta. Paediatr Scand [Suppl] 337: 118–121
Spivak JL, Hogans BB (1989) The in vivo metabolism of recombinant human erythropoietin in dialysis patients. Blood 73:90–99
Lim VS, DeGowin RL, Zavala D, Kirchner PT, Abels R, Perry P, Fangman J (1989) Recombinant human erythropoietin treatment in pre-dialysis patients. Ann Med 110:108–114
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Braun, A., Ding, R., Seidel, C. et al. Pharmacokinetics of recombinant human erythropoietin applied subcutaneously to children with chronic renal failure. Pediatr Nephrol 7, 61–64 (1993). https://doi.org/10.1007/BF00861571
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00861571