Conclusions
-
1.
A model was proposed for calculating the proton affinity of polyatomic anions.
-
2.
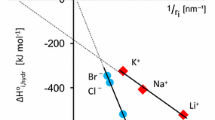
The proton affinity of the ions OmXO(OH) 1−n− decreases with increasing electronegativity of the atom X and with increasing number of oxygens surrounding the central atom.
Similar content being viewed by others
Literature cited
R. Gillespie, J. Chem. Soc., 2537 (1950).
L. Pauling, General Chemistry, San Francisco (1947), p. 394.
R. P. Bell, The Proton in Chemistry, London (1959).
A. Kossiakoff and D. Harker, J. Amer. Chem. Soc.,60, 2047 (1938).
I. E. Ricci, J. Amer. Chem. Soc.,70, 109 (1948).
J. P. Platt, J. Chem. Phys.,18, 932 (1950).
J. R. Platt, Handbuch der Physik, 37/2, S. Flugge, Springer-Verlag, Berlin (1961), p. 240.
W. G. McDuble and T. L. Brown, J. Amer. Chem. Soc.,89, 3111 (1967).
Yu. A. Borisov and N. N. Bulgakov, Izv. Akad. Nauk SSSR, Ser. Khim., 1606 (1971).
Yu. A. Borisov, N. N. Bulgakov, and A. E. Borisov, Izv. Akad. Nauk SSSR, Ser. Khim., 1480 (1969).
I. V. Abarenkov and V. Heine, Philos. Mag.,12, 529 (1965).
P. Gombash, The Many-Particle Problem in Quantum Mechanics [Russian translation], IL (1952).
V. I. Vedeneev, L. V. Gurevich, V. N. Kondrat'ev, V. A. Medvedev, and E. L. Frankevich, Cleavage Energies of Chemical Bonds. Ionization Potentials and Electron Affinity, Handbook [in Russian], Izd-vo AN SSSR (1962).
L. E. Sutton (editor), Tables of Interatomic Distances and Configurations in Molecules and Ions, London (1958).
C. Coulson, Valency [Russian translation], Mir (1965), p. 204.
J. C. Slater, J. Chem. Phys.,41, 3199 (1964).
J. P. Fischer, Trans. Faraday Soc.,64, 1852 (1968).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 1, pp. 18–23, January, 1972.
Rights and permissions
About this article
Cite this article
Borisov, Y.A., Bulgakov, N.N. Calculation of the proton affinity of anions of hydroxy acids. Russ Chem Bull 21, 16–20 (1972). https://doi.org/10.1007/BF00855647
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00855647