Conclusions
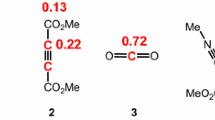
Electron-donor substituents (-OR, -OCOR, -SR) attached to an ethylenic carbon facilitate progress of the ionic hydrogenation reaction, while electron-acceptor substituents (-NO2,-COOH) prevent the progress of this reaction.
Similar content being viewed by others
Literature cited
Z. N. Parnes, V. I. Zdanovich, E. E. Kugucheva, G. I. Basova, and D. N. Kursanov, Dokl. Akad. Nauk SSSR,166, 122 (1966).
D. N. Kursanov, Z. N. Parnes, G. I. Bassova, N. M. Loim, and V. I. Zdanovich, Tetrahedron,23, 2235 (1967).
Z. N. Parnes, G. A. Khotimskaya, M. Yu. Lukina, and D. N. Kursanov, Dokl. Akad. Nauk SSSR,178, 620 (1968).
W. H. Mueller, Angew. Chem.,8, 482 (1969).
A. Lambert and A. Lowe, J. Chem. Soc., 1517 (1947).
N. Kornblum, B. Taub, and H. E. Ungnade, J. Am. Chem. Soc.,76, 3209 (1954).
N. Barbier and T. Léser, Bull. Soc. Chim. France,33, 815 (1905).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 9, pp. 1987–1989, September, 1972.
Rights and permissions
About this article
Cite this article
Parnes, Z.N., Bolestova, G.I. & Kursanov, D.N. Effect of substituents on the ionic hydrogenation reaction of olefins. Russ Chem Bull 21, 1927–1929 (1972). https://doi.org/10.1007/BF00854607
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00854607