Conclusions
-
1.
The conformational state of 1,1,1,3-tetrachloropropane was investigated by the PMR method in 11 solvents in a broad range of variation of the dielectric constant of the medium.
-
2.
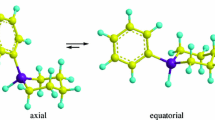
At 34°, the 1,1,1,3-tetrachloropropane molecule exists in solution under conditions of rapid rotation around the C2-C3 bond primarily in the trans-form, characterized by minimum dipole-dipole and steric interactions of the CCl3 group and chlorine atom. Some changes in the geminal constants and the sum of the vicinal constants were detected with increasing polarity of the solvent.
Similar content being viewed by others
Literature cited
B. A. Énglin, T. A. Onishchenko, Yu. V. Belov, V. I. Dostovalova, I. K. Shmyrov, and U. Utebaev, Izv. Akad. Nauk SSSR, Ser. Khim., 982 (1973).
S. Midzusima, Molecular Structure and Internal Rotation [Russian translation], IL (1957).
N. S. Gutowsky, G. G. Belford, and P. E. McMahon, J. Chem. Phys.,36, 3353 (1962).
R. J. Abraham and K. G. R. Pachler, Mol. Phys.,7, 165 (1964).
R. J. Abraham, L. Cavalli, and K. G. R. Pachler, Mol. Phys.,11, 471 (1966).
R. J. Abraham and G. Gatty, J. Chem. Soc., B, 961 (1969).
G. Govil and H. J. Bernstein, J. Chem. Phys.,48, 285 (1968); S. Kondo, E. Tagami, K. Limura, and M. Takeda, Bull. Chem. Soc. Japan,41, 790 (1968); W. F. Reynolds and D. J. Wood, Canad. J. Chem.,47, 1295 (1969);49, 1209 (1971); D. A. Dowson and W. F. Reynolds, ibid.,49, 3438 (1971).
B. Dischler and G. Englert, Z. Naturforsch.,16, 1180 (1961).
S. Sykora, Coll. Czechoslov. Chem. Comm.,33, 3514 (1968); P. E. McMahon, Trans. Faraday Soc.,61, 197 (1965).
E. B. Whipple, J. Magnetic Resonances,5, 163 (1971).
N. Sheppard and J. J. Turner, Proc. Roy. Soc., A252, 506 (1959); Y. Muroga, J. Noda, and M. Nagasawa, J. Phys. Chem.,73, 667(1969).
M. Karplus, J. Amer. Chem. Soc.,85, 287 (1963).
W. Lin, J. Chem. Phys.,50, 1890 (1969).
A. B. Dempster, K. Price, and N. Sheppard, Spectrochim. Acta,A27, 1563 (1971).
V. F. Bystrov, Uspekhi Khimii,41, 512 (1972).
G. M. Whitesides, J. P. Sevenair and R. W. Goetz, J. Amer. Chem. Soc.,89, 1135 (1967).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 5, pp. 989–995, May, 1973.
The authors are grateful to E. I. Matrosov and G. M. Petov for their useful discussion and S. M. Chernyak for measuring the dielectric permiability.
Rights and permissions
About this article
Cite this article
Énglin, B.A., Onishchenko, T.A., Dostovalova, V.I. et al. Conformational analysis of 1,1,1,3-tetrachloropropane by the pmr method. Russ Chem Bull 22, 954–959 (1973). https://doi.org/10.1007/BF00854230
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00854230