Summary
The movements of blastomere surfaces marked with carbon particles during cytokinesis of the Ist–IVth cleavage divisions in the eggs of the gastropodsLymnaea stagnalis, L. palustris, Physa acuta and Ph. fontinalis have been studied by time-lapse cinematographic methods. The vitelline membrane was removed with trypsin. At 2- and 4-cell stages shifts of nuclei have also been studied.
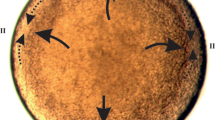
Symmetrical as well as asymmetrical surface movements (in respect to the furrow plane) have been revealed.
Symmetrical surface movements at the beginning of cytokinesis consist mainly in contraction of the furrow zone and in expansion of the more peripheral regions; between these there is a stationary zone. After the end of cytokinesis the furrow region expands.
Considerableasymmetrical surface movements have also been observed in all four divisions. From anaphase until the end of cytokinesis each of the two sister blastomeres rotates with respect to the other in such a way, that if viewed along the spindle axis, the blastomere nearest to the observer rotates dexiotropically in a dextral species and laeotropically in a sinistral species (primary rotations). After the completion of cytokinesis the blastomeres may rotate in a reverse direction. The latter rotations are less pronounced in the IInd and IIIrd divisions and most pronounced in the IVth division. Blastomeres with the vitelline membrane intact retain a slight capacity for primary rotations. In normal conditions nuclei of the first two blastomeres shift mainly laeotropically in dextral species, but dexiotropically in sinistral species, being carried along by the reverse surface rotations.
The invariable primary asymmetrical rotations of blastomeres seem to be the basis of enantiomorphism in molluscan cleavage. They are assumed to be determined by an asymmetrical structure of the contractile ring carrying out the cytokinesis.
Similar content being viewed by others
References
Bluemink, J. G.: The subcellular structure of the blastula ofLymnaea stagnalis L. (Mollusca) and the mobilization of the nutrient reserve. Thesis, Utrecht University, Rotterdam (1967)
Boycott, A. E., Diver, C., Garstang, S. L., Turner, F. M.: The inheritance of sinistrality inLymnaea peregra (Mollusca, Pulmonata). Phil. Trans. B219, 51–131 (1931)
Cather, J. N.: Cellular interactions in the regulation of development in annelids and molluscs. Advanc. Morphogenes.9, 67–125 (1971)
Conklin, Ed. G.: Embryology ofCrepidula. J. Morph.13, 1–127 (1897)
Conklin, Ed. G.: Karyokinesis and cytokinesis in the maturation, fertilization and cleavage ofCrepidula and other Gasteropoda. J. Acad. Nat. Sci. Phil.12, 1–121 (1902)
Conklin, Ed. G.: The cause of inverse symmetry. Anat. Anz.23, 577–588 (1903)
Dan, K.: On “the stationary surface ring” in heart-shaped cleavage. J. exp. Biol.35, 400–406 (1958)
Dan, K., Dan, J. C.: Behavior of the cell surface during cleavage. IV. Polar lobe formation and cleavage of the eggs ofIlyanassa obsoleta Say. Cytologia (Tokyo)12, 246 (1942)
Dan, K., Yanagita, T., Sugiyama, M.: Behavior of the cell surface during cleavage. I. Protoplasma28, 66–81 (1937)
Guerrier, P.: Nouvelles données expérimentales sur la segmentation et l'organogenèse chezLimax maximus (gastéropode pulmoné). Ann. embryol. et morphogenèse3, 283–294 (1970)
Guerrier, P.: La polarization cellulaire et les caractères de la segmentation au cours de la morphogenèse spirale (Gastéropodes pulmonés, Lamellibranches, Annélides polychètes). Ann. Biol.10, 151–192 (1971)
Hess, O.: Die Entwicklung von Halbkeimen bei dem Süßwasser-PulmonatenLimnaea stagnalis L. Wilhelm Roux' Arch. Entwickl.-Mech. Org.150, 124–145 (1957)
Meshcheryakov, V. N., Beloussov, L. V. (in Russian): Changes in the spatial organization of early cleavage of molluscsLymnaea stagnalis L. andPhysa fontinalis L. under the effect of trypsin. Ontogenesis4, 359–372 (1973)
Morrill, J. B., Perkins, F. O.: Microtubules in the cortical region of the egg ofLymnaea during cortical segregation. Develop. Biol.33, 206–212 (1973)
Rappaport, R.: Cytokinesis in animal cells. Int. Rev. Cytol.31, 169–218 (1971)
Raven, C. P.: Morphogenesis: the analysis of molluscan development, London-New York-Paris-Los Angeles: Pergamon Press 1958
Raven, C. P.: Determination of the direction of spiral coiling inLymnaea peregra. Acta morph. neerl.-scand.10, 165–178 (1972)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meshcheryakov, V.N., Beloussov, L.V. Asymmetrical rotations of blastomeres in early cleavage of gastropoda. Wilhelm Roux' Archiv 177, 193–203 (1975). https://doi.org/10.1007/BF00848080
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00848080