Summary
-
1.
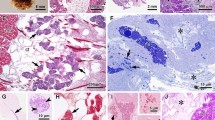
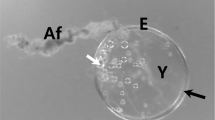
The egg ofPollicipes polymerus, the common intertidal gooseneck barnacle, has been studied by electron microscopy. Constriction rings, similar to the contractile rings of cleaving cells and polar lobes, move unidirectionally from the animal to the vegetal pole of newly fertilized eggs. This is referred to as peristaltic constriction. The present paper describes the fine structure of the egg during first polar body formation and peristalsis.
-
2.
During formation of the polar body, dense bodies are produced by the Golgi and extracellular plaques are observed. Thin microfilaments (40–60 Å) are in the egg adjacent to the polar body.
-
3.
In eggs undergoing peristalsis, the appearance of extracellular spheres, flocculent material and filaments is observed. Intracellularly large numbers of multivesiculate bodies, glycogen granules, mitochondria and protein-carbohydrate and lipid yolk bodies are seen at the level of constriction.
-
4.
Thin microfilaments are found in the cortical area of newly-fertilized eggs exclusively in peristaltic constriction rings. Filaments are oriented primarily in a meshwork, although circumferentially-oriented filaments are also found in rings near the vegetal pole. Microvilli extend into the space created between a constriction and the elevated egg membrane.
-
5.
A model is proposed to explain the peristalsis in this species. It is suggested that information from a pacemaker region activates peristalsis by affecting filament polymerization and orientation. One function of peristalsis may be elongation of the egg from a sphere to an ovoid, although other possibilities such as elevation of the egg membrane, segregation of the lipid yolk to the vegetal pole and predetermination of the first cleavage plane are also discussed.
Similar content being viewed by others
References
Allen, N.S.: Endoplasmic filaments generate the motive force for rotational streaming inNitella. J. Cell Biol.63, 270–287 (1974)
Anderson, E., Lochhead, J.H., Huebner, E.: The origin and structure of the tertiary envelope in thick shelled eggs of the brine shrimp.Artemia. J. Ultrastruct. Res.32, 497–525 (1970)
Arnold, J.M.: Cleavage furrow formation in a telolecithal egg (Loligo pealii). I. Filaments in early furrow formation. J. Cell Biol.41, 894–904 (1969)
Arnold, J.M.: Cleavage furrow formation in a telolecithal egg (Loligo pealii). II. Direct evidence for a contraction of the cleavage furrow base. J. Exp. Zool176, 73–85 (1971)
Barnes, H.: Studies in the biochemistry of cirripede eggs. I. Changes in the general biochemical composition during development ofBalanus balanoides (L.) andBalanus balanus da Costa. J. Mar. Biol. Assoc.45, 321–339 (1965)
Barnes, H., Evens, R.: Studies in the biochemistry of cirripede eggs. III. Changes in the amino acid composition during development ofBalanus balanoides andBalanus balanus. J. Mar. Biol. Assoc.47, 171–180 (1967)
Bluemink, J.G.: The first cleavage of the amphibian egg—an electron microscope study of the onset of cytokinesis in the egg ofAmbystoma mexicanum. J. Ultrastruct. Res.32, 142–166 (1970)
Bluemink, J.G.: Cytokinesis and cytochalasin-induced furrow regression in the first-cleavage zygote ofXenopus laevis. Z. Zellforsch121, 102–126 (1971)
Byers, B., Porter, K.R.: Oriented microtubules in elongating cells of the developing lens rudiment after induction. Proc. Nat. Acad. Sci. U.S.52, 1091–1099 (1964)
Cande, W.Z., Lazarides, E., McIntosh, J.R.: Visualization of actin in functional lysed mitotic cell preparations. J Cell Biol.67, 54a (1975)
Cloney, R.A., Florey, E.: Ultrastructure of cephalopod chromatophore organs. Z. Zellforsch.89, 250–280 (1968)
Conrad, G.W., Williams, D.C.: Polar lobe formation and cytokinesis in fertilized eggs ofIlyanassa obsoleta. II. Large bled formation caused by high concentrations of exogenous calcium ions. Dev. Biol.37, 280–295 (1974)
Conti, C.J., Klein-Szanto, A.J.P.: Nuclear multivesicular bodies in cultured hamster cells. Experientia29, 850–851 (1973)
Crowell, J.: The fine structure of the polar lobe ofHyanassa obsoleta. Acta Embryol. Morph. exp.7, 225–234 (1964)
Dawson, R.M.C., Barnes, H.: Studies in the biochemistry of cirripede eggs. II. Changes in lipid composition during development ofBalanus balanoides andBalanus balanus. J. Mar. Biol. Assoc.46, 249–261 (1966)
Dohmen, M.R., Lok, D.: The ultrastructure of the polar lobe ofCrepidula fornicata (Gastropoda, Prosobranchia). J. Embryol. exp. Morph.34, 419–438 (1975)
Eastman, R.C.: Activities of several pentose shunt and glycolytic enzymes in developing eggs of the barnacle,Pollicipes polymerus. Exp. Cell Res51, 323–329 (1968)
Frey-Wyssling, A.:Submicroscopic Morphology of Protoplasm pp. 411. Amsterdam: Elsevier Pub. Co. 1953
Groom, T.T.: On the early development ofCirripedia. Phil. Trans. Roy. Soc. London52, 158–162 (1892)
Gruenstein, E., Rich, A., Weihing, R.R.: Actin associated with membranes from 3T3 mouse fibroblast and HeLa cells. J. Cell Biol.64, 223–234 (1975)
Hara, K.: Cinematographic observation of “surface contraction waves” (SCW) during the early cleavage ofAxolotl eggs. Wilhelm Roux' Archiv167, 183–186 (1971)
Kamiya, N.: Physics and chemistry of protoplasmic streaming. Ann. rev. Plant Physiol.11, 323–340 (1960)
Kang, Y.-H.: Development of the zona pellucida in the rat oocyte. Am. J. Anat.139, 535–566 (1974)
Komnick, H., Stockem, W., Wohlfarth-Bottermann, E.: Cell mobility: mechanisms in protoplasmic streaming and amoeboid movement. Inter. Rev. Cytol.34, 169–249 (1973)
Lewis, C.A.: Development of the gooseneck barnaclePollicipes polymerus (Cirripedia: Lepadomorpha): Fertilization through settlement. Mar. Biol.32, 141–153 (1975)
Lewis, C.A., Chia, F.-S., Schroeder, T.E.: Peristaltic constrictions in fertilized barnacle eggs (Pollicipes polymerus). Experientia29, 1533–1535 (1973)
Locke, M., Collins, J.V.: Protein uptake in multivesicular bodies in the molt-intermolt cycle of an insect. Science155, 467–469 (1967)
Luft, J.H.: Improvements in epoxy resin embedding methods. J. Biophys. Biochem. Cytol.9, 409–414 (1961)
Millonig, G.: Advantages of a phosphate buffer for osmium tetroxide solutions in fixation. J. Applied Physics32, 1637 (1961)
Miranda, A.F., Godman, G.C., Tanenbaum, S.W.: Action of cytochalasin D on cells of established lines. II. Cortex and microfilaments. J. Cell Biol.62, 406–423 (1974)
Mooseker, M.S., Tilney, L.G.: Organization of an actin filament-membrane complex. Filament polarity, and membrane attachment in the microvilli of intestinal epithelial cells. J. Cell Biol.67, 725–743 (1975)
Morill, J.B., Perkins, F.O.: Microtubules in the cortical region of the egg ofLymnaea during cortical segregation. Dev. Biol.33, 206–212 (1973)
Orci, L., Stauffacher, W.: Glycogenosomes in renal tubular cells of diabetic animals. J. Ultrastruct Res.36, 499–503 (1971)
Pollard, T.D., Weihing, R.R.: Actin and myosin and cell movement. CRC Crit. Rev. Biochem.2, 1–65 (1974)
Porter, K.R.: Microtubules in intracellular locomotion. In:Ciba Foundation Symposium 14, Locomotion of Tissue Cells, p. 149–169. New York: Elsevier 1973
Perry, M.M.: Identification of glycogen in thin sections of amphibian embryos. J. Cell Sci2, 257–264 (1967)
Reynolds, E.S.: The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J. Cell Biol.17, 208–212 (1963)
Richardson, K.C., Jarrett, L., Finke, E.H.: Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Tech.35, 313–323 (1960)
Robbins, E., Gonatas, N.K.: Histochemical and ultrastructural studies on HeLa cell cultures exposed to spindle inhibitors with special reference to the interphase cell. J. Histochem. Cytochem.12, 704–711 (1964a)
Robbins, E., Gonatas, N.K.: Ultrastructure of a mammalian cell during the mitotic cycle. J. Cell Biol.21, 429–463 (1964b)
Schroeder, T.E.: Cytokinesis: filaments in the cleavage furrow. Exp. Cell Res.,53, 272–276 (1968)
Schroeder, T.E.: The contractile ring: I. Fine structure of dividing mammalian (HeLa) cells and the effects of cytochalasin B. Z. Zellforsch.109, 431–449 (1970)
Schroeder, T.E.: Mechanisms of morphogenesis: the embryonic neural tube. Inter. J. Neuroscience2, 183–198 (1971)
Schroeder, T.E.: Actin in dividing cells: contractile ring filaments bind heavy meromyosin. Proc. Nat. Acad. Sci. U.S.70, 1688–1692 (1973)
Szollosi, D.: Cortical cytoplasmic filaments of cleaving eggs: a structural element corresponding to the contractile ring. J. Cell Biol.44, 192–209 (1970)
Szubinska, B.: “New membrane” formation inAmoeba proteus upon injury of individual cells, electron microscope observations. J. cell Biol.49, 747–772 (1971)
Tilney, L.G., Marsland, D.: A fine structural analysis of cleavage induction and furrowing in the eggs ofArbacia punctulata. J. Cell Biol42, 170–184 (1969)
Walley, L.J., White, F., Brander, K.M.: Sperm activation and fertilization inBalanus balanoides. J. Mar. Biol. Assoc.51, 489–494 (1971)
Woods, J.H.: Histochemistry and ultrastructure of oogenesis inIbla quadrivalvis andTetraclita rosea and the biochemical composition of developing embryos ofIbla quadrivalvis (Crustacea, Cirripedia). M.S. Thesis. Univ. of Sydney, Australia 1969 (Unpublished)
Woollacott, R.M.: Microfilaments and mechanism of light-triggered sperm release in ascidians. Dev. Biol.40, 186–195 (1974)
Yamada, K.M., Wessells, N.K.: Cytochalasin B: effects on membrane ruffling, growth cone and microspike activity, and microfilament structure not due to altered glucose transport. Dev. Biol.31, 413–420 (1973)
Zerbib, C.: First observation of cortical granules in a crustacean, the amphipodOrchestia gammarellus (Pallas). Comp. Rend.281, 1347–1349 (1975)
Zissler, D., Sander, K.: The cytoplasmic architecture of the egg cell ofSmittia sp. (Diptera, Chironomidae). I. Anterior and posterior pole regions. Wilhelm Roux' Archiv.172, 175–186 (1973)
Author information
Authors and Affiliations
Additional information
I thank Dr. Robert Fernald, former Director of the Friday Harbor Laboratories: Dr. Cadet Hand, Director of the Bodega Bay Marine Station; and Dr. David Pawson, Head of the Invertebrate Zoology Department Smithsonian Institution for use of research facilities. I am grateful to Dr. F.S. Chia, Dr. S.C. Chang, Dr. W.D. Hope, Dr. K. Yamada, Dr. J. Hassell Dr. R. Greene and Dr. H. Reddi for reading the manuscript and offering suggestions for its improvement. This investigation was supported by a grant to Dr. F.S. Chia and a postgraduate scholarship to C. Lewis, both provided by the National Research Council of Canada
Rights and permissions
About this article
Cite this article
Lewis, C.A. Ultrastructure of a fertilized barnacle egg (Pollicipes polymerus) with peristaltic constrictions. Wilhelm Roux' Archiv 181, 333–355 (1977). https://doi.org/10.1007/BF00848060
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00848060