Summary
-
1.
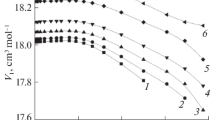
The size of the elementary surface area occupied by a N2 molecule adsorbed on Al2O3 is almost independent of the degree of surface hydration of the adsorbent.
-
2.
The degree of surface hydration of Al2O3 noticeably affects the elementary surface area occupied by a C2H5OH molecule, but this effect is not so great that it might explain the variations which we have previously observed in the values ofω for one and the same alcohol adsorbed on Al2O3 samples of different origin.
-
3.
Theω values of C6H12 and C5H12 on the samples examined are not outside the physically real range, although interaction forces may arise between the adsorbate molecules, which might considerably increase the apparent values ofω.
Similar content being viewed by others
Literature cited
V. E. Vasserberg, A. A. Balandin, and M. P. Maksimova, Izv. AN SSSR, Otd. Khim, n.1959, 363.
V. E. Vasserberg, A. A. Balandin, and M. P. Maksimova. Zh. fiz. khimii35, 858 (1961).
I. B. Peri and R. B. Hannan, J. Phys. Chem.64, 1526 (1960).
M. I. Egorov, T. S. Egorova, K. G. Krasil'nikov, and V. F. Kiselev, Zh. fiz. khimii 32, 2624 (1958).
A. A. Isirikyan and A. V. Kiselev, Dokl. AN SSSR115, 343 (1957).
A. M. Rubinshtein, Yu. A. El'tekov, and T. R. Brueva, Izv. AN SSSR, Otd. khim. n.1960, 2107.
M. K. Cerrin, J. Amer. Chem. Soc.73, 4061 (1951).
K. G. Krasil'nikov, V. F. Kiselev, N. V. Kapitonova, and E. A. Sysoev, Zh. fiz. khimii31, 1448 (1957).
E. B. Kornelius, T. H. Millikan, G. A. Mills, and A. G. Oblad, J. Phys. Chem.59, 809 (1955).
M. M. Dubinin, E. G. Zhukovskaya, E. D. Zaverina, I. E. Neimark, and R. Yu. Sheinfain, Izv. AN SSSR, Otd. khim. n.1960, 588.
L. G. Granichenko, M. M. Dubinin, E. D. Zaverina, V. F. Kiselev, and K. G. Krasil'nikov, Izv. AN SSSR, Otd. khim. n.1960, 1535.
M. M. Dubinin, Izv. AN SSSR, Otd. khim. n.1960, 1739.
V. E. Vasserberg, A. A. Balandin, and T. V. Georgievskaya, Dokl. AN SSSR140, 859 (1961).
O. V. Krylov and E. A. Fokina, Kinetika i kataliz1, No. 4, 542 (1960).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maksimova, M.P., Vasserberg, V.E. & Balandin, A.A. Effect which the degree of hydration of the Al2O3 surface has on the adsorptive properties and the areas occupied by adsorbed molecules. Russ Chem Bull 12, 13–16 (1963). https://doi.org/10.1007/BF00846942
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00846942