Summary
-
1.
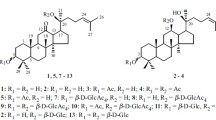
By the condensation of acetobromoglucose with methyl oleanolate in presence of mercuric acetate and with 3-O-acetyloleanolic acid in presence of silver carbonate methyl oleanolate α-D-glucoside and 1-(3-O-acetyl-oleanoloyl)-β-D-glucopyranose were obtained, respectively.
-
2.
By the halolysis of methyl oleanolate α- andβ -glucosides with lithium iodide in collidine, oleanolic acid 3-O-α- and 3-O-β -glucosides were obtained, respectively, which indicates the stability of a glycoside link of the usual type under the conditions of the halolysis reaction.
-
3.
With 1-(3-O-acetyloleanoloyl)-β -D-glucose and araloside A as examples, the possibility of the selective cleavage of the acyl glycoside link in halolysis was demonstrated.
Similar content being viewed by others
Literature cited
N. K. Kochetkov, A. Ya. Khorlin, and V. E. Vas'kovskii, Izv. AN SSSR, Otd. khim. n.,1963, 1398.
N. K. Kochetkov, A. Ya. Khorlin, and V. E. Vas'kovskii, Izv. AN SSSR, Otd. khim. n.,1963, 1409.
N. K. Kochetkov, A. Ya. Khorlin, and Yu. S. Ovodov, Izv. AN SSSR, Ser. khim.,1964, 90.
N. K. Kochetkov, A. Ya. Khorlin, and Yu. S. Ovodov, Izv. AN SSSR, Ser. khim.,1964, 1436.
A. Ya. Khorlin and A. G. Ven'yaminova, Izv. AN SSSR, Ser. khim.,1964, 1447.
A. Ya. Khorlin and V. M. Ivanova, Aptechnoe delo,1963, No. 6, 31.
J. Polonski, E. Sach, and E. Lederer, Bull. Soc. Chim. France, 880 (1959).
E. Hardegger and F. Robinet, Helv. chim. acta,33, 1871 (1950).
E. Hardegger, F. Robinet, and A. Leemann, Helv. chim. acta,35, 824 (1952).
E. Hardegger and J. de Pascual Teresa, Helv. chim. acta,31, 281 (1948).
E. Taschner and B. Ziberek, Rochniki chemii,30, 323 (1956).
F. Elsinger, J. Schreiber, and A. Eschenmoser, Helv. chim. acta,43, 113 (1960).
R. A. Lucas, D. F. Dickel, R, L. Driemian, M. J. Geglowski, B. L. Hensle, and H. B. MacPhillamy, J. Am. Chem. Soc.,82, 5688 (1960).
J. V. Euw, A. Lardon, and T. Reichstein, Helv. chim. acta,27, 1292 (1944).
E. Stahl, Arch. parm.,292, 411 (1959).
A. Ya. Khorlin, L. V. Bakinovskii, V. E. Vas'kovskii, A. G Ven'yamina, and Yu. S. Ovodov, Izv. AN SSSR, Ser. khim.,1963, 1208.
N. K. Kochetkov, A. Ya. Khorlin, and A. F. Bochkov, DAN SSSR,136, 613 (1961).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya No. 11, pp. 2028–2036, November, 1964