Summary
-
1.
It is pointed out that in the general case of aerosol filtration, the escape coefficient and the resistance of the filter change with time. Accordingly, in addition to the primary filtration factors resulting from diffusion, direct capture, inertia, etc., it is a good idea to introduce the concept of secondary processes which produce changes in properties of the filter during absorption of the aerosol.
-
2.
The secondary filtration factors include coprecipitation of particles in the filter, capillary phenomena, and destruction of the filter, as well as electric charge losses in the filtering material. The effect of these factors is to produce a change in the escape coefficient and the resistance of the filter with time.
-
3.
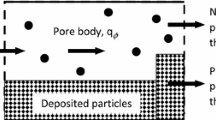
The simplest theory is given for the precipitation of particles on top of one another in filtration, based on die assumption that the capture cross-section of die particles is constant, and that the filter operates in layers. Differential equations are derived for the kinetics of the coprecipitation process, and the resulting solutions give expressions for the escape coefficient, and for the number of precipitated particles as a function of the time and of the thickness of the layer.
Similar content being viewed by others
Literature cited
E. Rodebush, Handbook on Aerosols, Washington (1950), p. 117; N. A. Fuks, Mechanics of Aerosols [in Russian], Academy of Sciences Press USSR, Moscow (1955), pp. 201–202.
G. Fairs, Trans. Instn. Chem. Engrs.36 476 (1958).
N. A. Fuks, ibid..
C. N. Davies, J. Instn. Heat. and Ventilat. Engrs.20 N 201, 55 (1952).
R. Leers, Staub, Vol. 50, 402 (1957).
D. Hasenclever, Staub19 37 (1959).
V. K. LaMera and V. G. Drozin, Proc. of the Second Internat. Congress of Surface Activity, III, London, Butterworths, Scientif. Public. (1957), pp. 600–606.
L. Dautrebande, E. Dumoulin, and P. Angenot, Compt. rend.156 240, 329 (1937).
V. S. Bondarenko, Zh. fiz. khimii35 2775 (1961); I. V. Petryanov and N. A. Rozenblyum, Dokl. AN SSSR61, 661(1948); G. Fairs, ibid..
N. A. Fuks, ibid..
A. T. Rossano and L. Silverman, Heating and Ventilating51 N 5, 101 (1954).
Ch. Chen, Uspekhi khimii25 No. 3, 368 (1956).
I. O. Irwin, P. Armitage, and C. N. Davies, Nature (London),163 809 (1949).
A. N. Tikhonov and A. A. Samarskii, Equations of Mathematical Physics [in Russian], Moscow (1953), pp. 127 and 163.
Yu. M. Shekhtman, Dokl. AN SSSR98 No. 4, 549 and No. 6, 917 (1954).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Radushkevich, L.V. The secondary processes in aerosol filtration. Russ Chem Bull 12, 367–372 (1963). https://doi.org/10.1007/BF00844384
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00844384