Abstract
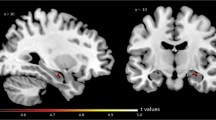
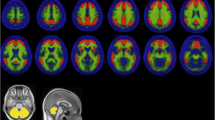
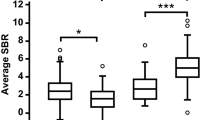
Decreased muscarinic receptor binding has been suggested in single-photon emission tomography (SPET) studies of Alzheimer's disease. However, it remains unclear whether these changes are present in mildly demented patients, and the role of cortical atrophy in receptor binding assessment has not been investigated. We studied muscarinic receptor binding normalized to neostriatum with SPET using [123I]4-iododexetimide in five mildly affected patients with probable Alzheimer's disease and in five age-matched control subjects. Region of interest (ROI) analysis was performed in a consensus procedure blind to clinical diagnosis using matched magnetic resonance (MRI) images. Cortical atrophy was assessed by calculating percentages of cerebrospinal fluid in each ROI. An observer study with three observers was conducted to validate this method. Alzheimer patients showed statistically significantly less [123I]4-iododexetimide binding in left temporal and right temporo-parietal cortex compared with controls, independent of age, sex and cortical atrophy. Mean intea-observer variability was 3.6% and inter-observer results showed consistent differences in [123I]4-iododexetimide binding between observers. However, differences between patients and controls were comparable among observers and statistically significant in the same regions as in the consensus procedure. Using an MRI-SPET matching technique, we conclude that [123I]4-iododexetimide binding is reduced in patients with mild probable. Alzheimer's disease in areas of temporal and temporoparietal cortex.
Similar content being viewed by others
References
Müller-Gärtner HW, Wilson AA, Dannals RF, et al. Imaging muscarinic cholinergic receptors in human brain in vivo with SPECT, [123I]4-iododexetimide, and [123I]4-iodolevetimide.J Cereb Blood Flow Metab 1992; 12: 562–570.
Weinberger DR, Gibson R, Coppola R, et al. The distribution of cerebral muscarinic acetylcholine receptors in vivo in patients with dementia. A controlled study with123IQNB and single photon emission computed tomography.Arch Neurol 1991; 48: 169–176.
Wyper DJ, Brown D, Patterson J, et al. Deficits in iodine-labelled 3-quinuclidinyl benzilate binding in relation to cerebral blood flow in patients with Alzheimer's disease.Eur J Nucl Med 1993; 20: 379–386.
Boundy KL, Rowe CC, Reid M, et al. Early diagnosis of Alzheimer's disease (AD) with SPECT imaging of muscarinic cholinergic neuroreceptors (mChR) using 1-123 iododexetimide (IDEX).Neurology 1995; 45 Suppl 4: A323-A324.
McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease.Neurology 1984; 34: 939–944.
Roth M, Huppert FA, Tym E, et al.CAMDEX, the Cambridge Examination for Mental Disorders of the Elderly. Cambridge: Cambridge University Press, 1988.
Derix MM, Hofstede AB, Teunisse S, et al. CAMDEX-N: the Dutch version of the Cambridge Examination for Mental Disorders of the Elderly with automatic data processing.Tijdschr Gerontol Geriatr 1991; 22: 143–150.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res 1975; 12: 189–198.
Lindeboom J, ter Horst R, Hooyer C, et al. Some psychometric properties of the CAMCOG.Psychol Med 1993; 23: 213–219.
Stoddart HF, Stoddard HA. A new development in single gamma transaxial tomography union carbide focussed collimator scanner.IEEE Trans Nucl Sci NS 1979; 26: 2710–2712.
van Herk M, Kooy H. Automatic three dimensional correlation of CT-CT, CT-MRI and CT-SPELT using chamfer matching.Med Phys 1994; 21: 1163–1178.
Aquilonius SM, Eckernas SA.A color atlas of the human brain. New York: Raven Press, 1980.
Claus JJ, van Harskamp F, Breteler MMB, et al. The diagnostic value of SPELT with Tc99m HMPAO in Alzheimer's disease: a population-based study.Neurology 1994; 44: 454–461.
Tomlinson BE. Aging and the dementias. In: Adams JH, Duchen LW, eds.Greenfield's neuropathology. London: Edward Arnold; 1992; 1284–1410.
BMDP statistical software manual. Berkeley: University of California Press, 1992.
Streiner DL, Norman GR.Health measurement scales. A practical guide to their development and use. Oxford: Oxford University Press, 1995; 104–127.
Podoll K, Caspary P, Lange HW, et al. Language functions in Huntington's disease.Brain 1988; 111: 1475–1503.
Tanna NK, Kohn MI, Horwich DN, et al. Analysis of brain and cerebrospinal fluid volumes with MR imaging: impact on PET data correction for atrophy. Part II. Aging and Alzheimer dementia.Radiology 1991; 178: 123–130.
Coleman PD, Flood DG. Neuron numbers and dendritic extent in normal aging and Alzheimer's disease.Neurobiol Aging 1987; 8: 521–545.
Creasey H, Schwartz M, Frederickson H, et al. Quantitative computed tomography in dementia of the Alzheimer type.Neurology 1986; 36: 1563–1568.
Meltzer CC, Zubieta JK, Brandt J, et al. Regional hypometabolism in Alzheimer's disease as measured by positron emission tomography after correction for effects of partial volume averaging.Neurology 1996; 47: 454–461.
Weinberger DR, Jones D, Reba RC, et al. A comparison of FDG PET and IQNB SPELT in normal subjects and in patients with dementia.J Neuropsychiatry Clin Neurosci 1992; 4: 239–248.
Claus JJ, van Gool WA, Feldman H, Mohr E. The clinical utility of brain SPELT in Alzheimer's disease: a critical view of the report by the Therapeutics and Technology Assessment Committee of the American Academy of Neurology (letter).Neurology 1997, in press.
Jobst KA, Shepstone BJ, Smith AD, et al. The importance of single photon emission computed tomography (SPELT) cerebral blood flow imaging to accurate diagnosis in histologically confirmed dementia.Acta Neurol Belg 1995; 95 Suppl: 5–18.
van Gool WA, Walstra GJM, Teunisse S, et al. Diagnosing Alzheimer's disease in elderly, mildly demented patients: the impact of routine single photon emission computed tomography.J Neurol 1995; 242: 401–405.
Caulfield MP, Straughan DW Cross AJ, et al. Cortical muscarinic receptor subtypes and Alzheimer's diseae.Lancet 1982; II: 1277.
Kellar KJ, Whitehouse PJ, Martino-Barrows AM, et al. Muscarinic and nicotinergic cholinergic binding sites in Alzheimer's disease cerebral cortex.Brain Res 1987; 436: 62–68.
Waller SB, Ball MJ, Reynolds MA, et al. Muscarinic binding and choline acetyltransferase in postmortem brains of demented patients.Can J Neurol Sci 1986; 13: 528–532.
Nordberg A, Nyberg P, Adolfsson R, et al. Cholinergic topography in Alzheimer brains: a comparison with changes in the monoaminergic profile.J Neurol Trans 1987; 69: 19–32.
Rinne JO, Laakso K, Lonnberg P, et al. Brain muscarinic receptors in senile dementia.Brain Res 1985; 336: 19–25.
Smith CJ, Perry EK, Perry RH, et al. Muscarinic cholinergic receptor subtypes in hippocampus in human cognitive disorders.J Neurochem 1988; 50: 847–856.
Nordberg A. Neuroreceptor changes in Alzheimer's disease.Cerebrovasc Brain Metab Rev 1992; 4: 303–328.
Bonner TI. The molecular basis of muscarinic receptor diversity.Trends Pharmacol Sci 1989; 10: 148–151.
Flynn DD, Ferrari-DiLeo G, Mash DC, et al. Differential regulation of molecular subtypes of muscarinic receptors in Alzheimer's disease.J Neurochem 1995; 64: 1888–1891.
Flynn DD, Weinstein DA, Mash DC. Loss of high-affinity agonist binding to M1 muscarinic receptors in Alzheimer's disease: implications for the failure of cholinergic replacement therapies.Ann Neurol 1991; 29: 256–262.
Jope RS, Song L, Li X, et al. Impaired phosphoinositide hydrolysis in Alzheimer's disease brain.Neurobiol Aging 1994; 15: 221–226.
Mouradian MM, Mohr E, Williams JA, et al. No response to high-dose muscarinic agonist therapy in Alzheimer's disease.Neurology 1988; 38: 606–608.
Harbaugh RE, Reeder TM, Senter HJ, et al. Intracerebroventricular bethanechol chloride infusion in Alzheimer's disease. Results of a collaborative double-blind study.J Neurosurg 1989; 71: 481–486.
Ehlert FJ, Roeske WR, Yamamura HI. Muscarinic receptors and novel strategies for the treatment of age-related brain disorders.Life Sci 1994; 55: 2135–2145.
Eagger SA, Levy R, Sahakian BJ. Tacrine in Alzheimer's disease.Lancet 1991; 337: 989–992.
Knapp MJ, Knopman DS, Solomon PR, et al. A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer's disease.JAMA 1994; 271: 985–991.
Davis KL, Thal LJ, Gamzu ER, et al. A double-blind, placebo-controlled multicenter study of tacrine for Alzheimer's disease.N Engl J Med 1992; 327: 1253–1259.
Farlow M, Gracon SI, Hershey LA, et al. A controlled trial of tacrine in Alzheimer's disease.JAMA 1992; 268: 2523–2529.
Roth GS, Joseph JA, Mason RP. Membrane alterations as causes of impaired signal transduction in Alzheimer's disease and aging.Trends Neurosci 1995; 18: 203–206.
Ferrari-DiLeo G, Mash DC, Flynn DD. Attenuation of muscarinic receptor-G-protein interaction in Alzheimer's disease.Mol Chem Neuropathol 1995; 24: 69–91.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Claus, J.J., Dubois, E.A., Booij, J. et al. Demonstration of a reduction in muscarinic receptor binding in early Alzheimer's disease using iodine-123 dexetimide single-photon emission tomography. Eur J Nucl Med 24, 602–608 (1997). https://doi.org/10.1007/BF00841396
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00841396