Abstract
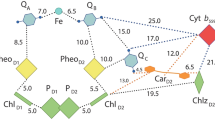
A review of a recent study of the spectral and thermodynamic properties of cytochrome b559 as well as of the electron transfer between b559 and photosystem II reaction center cofactors in isolated D1/D2/cytochrome b559 complex RC-2 is presented. Attention is paid to the existence of intermediary-potential (IP, +150 mV) and extra-low-potential (XLP, −45 mV) hemes located close to the acceptor (quinone) and donor (P680) sides of the reaction center cofactors, respectively. These hemes found in isolated RC-2 probably correspond to the high-potential and low-potential hemes in chloroplasts, respectively. The above location of the hemes is believed to allow the photoreduction of the XLP heme and photooxidation of the IP heme. The electron transfer between the two hemes is discussed in terms of the cyclic electron flow and possible involvement in water splitting.
Similar content being viewed by others
References
Babcock, G. T., Widger, W. R., Cramer, W. A., Oertling, W. A., and Metz, J. G. (1985).Biochemistry 24 3638–3645.
Barber, J., and de Las Rivas, J. (1993).Proc. Natl. Acad. Sci. USA 90 10942–10946.
Blumberg, W. E., and Peisach, J. (1971). InProbes of Structure and Function of Macromolecules and Membranes (Chance, B., Yonetani, T., and Mildvan, A. S., eds.), Academic Press, New York, Vol. 2, pp. 215–228.
Buser, C. A., Diner, B. A., and Brudvig, G. M. (1992).Biochemistry 31 11441–11459.
Chapman, D. J., Gounaris, K., and Barber, J. (1988).Biochim. Biophys. Acta 933 423–431.
Cox, R. P., and Bendall, D. S. (1972).Biochim. Biophys. Acta 283 124–135.
Cramer, W. A., and Butler, W. L. (1967).Biochim. Biophys. Acta 143 332–339.
Cramer, W. A., and Whitmarsh, J. (1977).Annu. Rev. Plant Physiol. 28 133–172.
Cramer, W. A., Theg, S. M., and Widger, W. R. (1986).Photosynth. Res. 10 393–403.
Debus, R. J. (1992).Biochim. Biophys. Acta 1102 269–352.
Deisenhofer, J., Epp, O., Miki, K., Huber, R., and Michel, H. (1985).Nature (London)318 618–624.
Erixon, K., Lozier, R., and Butler, W. L. (1972).Biochim. Biophys. Acta 267 375–382.
Fujita, I., Davies, M. S., and Fajer, J. (1978).J. Am. Chem. Soc. 100 6280–6282.
Gounaris, K., Chapman, D. J., and Barber, J. (1988).FEBS Lett. 240 143–147.
Heber, U., Kirk, M. R., and Boardman, N. K. (1979).Biochim. Biophys. Acta 546 292–306.
Horton, P., Whitmarsh, J., and Cramer, W. A. (1976).Arch. Biochem. Biophys. 176 519–524.
Kaminskaya, O. P., and Shuvalov, V. A. (1994).FEBS Lett., submitted.
van Kan, P. J. M., Otte, S. C. M., Kleinherenbrik, F. A. M., Nieveen, M. C., Aartsma, T. J., and van Gorkom, H. J. (1990).Biochim. Biophys. Acta 1020 146–152.
Matsuda, H., and Butler, W. L. (1983).Biochim. Biophys. Acta 724 123–127.
Michel, H., and Deisenhofer, J. (1988).Biochemistry 27 1–7.
Nanba, O., and Satoh, K. (1987).Proc. Natl. Acad. Sci. USA 84 109–112.
Parkasi, H. B., and Wermaas, W. F. J. (1992). InThe Photosystems: Structure, Function and Molecular Biology (Barber, J., ed.), Elsevier, Amsterdam, pp. 231–257.
Satoh, K., Hansson, O., and Mathis, P. (1970).Biochim. Biophys. Acta 1016 121–126.
Schonbaum, G. R., and Chance, B. (1976). InThe Enzymes (Boyer, P. D., ed.), Vol. 13, Part C, pp. 363–408.
Shuvalov, V. A., and Kaminskaya, O. P. (1993). In Abstracts of Topical ESF workshop “Spectroscopy of isolated D1D2 reaction centers,” p. 1, Max-Planck-Inst. fur Strahlenchemie, Mulheim.
Shuvalov, V. A., Heber, U., and Schreiber, U. (1989).FEBS Lett. 258 27–31.
Shuvalov, V. A., Schreiber, U., and Heber, U. (1994a).FEBS Lett. 337 226–230.
Shuvalov, V. A., Fiege, R., Schreiber, U., Lendzian, F., and Lubitz, W. (1994b).Biochim. Biophys. Acta, to be submitted.
Takahashi, Y., Hansson, O., Mathis, P., and Satoh, K. (1987).Biochim. Biophys. Acta 893 49–59.
Vallon, O., Tae, G.-S., Cramer, W. A., Simpson, D., Hoyer-Hansen, G., and Bogorad, L. (1989).Biochim. Biophys. Acta 975 132–141.
Widger, W. R., Cramer, W. A., Hermodson, M., and Herrmann, R. G. (1985).FEBS Lett. 191 186–190.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shuvalov, V.A. Composition and function of cytochrome b559 in reaction centers of photosystem II of green plants. J Bioenerg Biomembr 26, 619–626 (1994). https://doi.org/10.1007/BF00831536
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00831536