Abstract
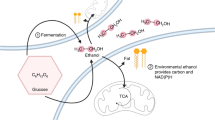
The regulation of the PQQ-linked glucose dehydrogenase in different organisms is reviewed. It is concluded that this enzyme functions as an auxiliary energy-generating mechanism, because it is maximally synthesized under conditions of energy stress. It is now definitively established that the oxidation of glucose to gluconate generates metabolically useful energy. The magnitude of the contribution of the oxidation of glucose to gluconate via this enzyme to the growth yield of organisms such asAcinetobacter calcoaceticus is not yet clear.
Similar content being viewed by others
References
Ameyama, M, Shinagawa, E, Matsushita, K, and Adachi, O, 1985. Growth stimulating activity for microorganisms in naturally occurring substances and partial characterization of the substance for the activity as PQQ. Agricultural and Biological Chemistry 49: 699–709.
Beardmore-Gray, M, and Anthony, C, 1986. The oxidation of glucose byAcinetobacter calcoaceticus: the interaction of the quinoprotein glucose dehydrogenase with the electron transport chain. Journal of General Microbiology 132: 1257–1268.
Boiardi JL, Buurman ET, Hardy GPMA, Teixeira de Mattos MJ, and Neijssel OM, 1988. The effect of magnesium and calcium on the synthesis of PQQ inKlebsiella aerogenes andPseudomonas species. Poster abstract, First International Symposium on PQQ and Quinoproteins, Delft.
Bont, JAMde, Dokter, P, Schie, BJvan, Dijken, JPvan, Frank Jzn, J, Duine, JA, and Kuenen, JG, 1984. Role of quinoprotein glucose dehydrogenase in gluconic acid production byAcinetobacter calcoaceticus. Antonie van Leeuwenhoek 50: 76–77.
Boutroux, L, 1880. Sur une fermentation nouvelle du glucose. Compters Rendus Hebdomadaires des Seances de l'Academie des Sciences 91: 236–238.
Campbell, JJR, Ramakrishna, T, Linnes, AG, and Eagles, BA, 1956. Evaluation of the energy gained byPseudomonas aeruginosa during the oxidation of glucose to 2-ketogluconate. Canadian Journal of Microbiology 2: 304–310.
Dalby, A, and Blackwood, AC, 1955. Oxidation of sugars by an enzyme preparation fromAerobacter aerogenes. Canandian Journal of Microbiology 1: 733–742.
Duine, JA, Frank Jzn, J, and Zeeland, JKvan, 1979. Glucose dehydrogenase fromAcinetobacter calcoaceticus: a quinoprotein. FEBS Letters 108: 443–446.
Hardy GPMA, Teixeira de Mattos MJ, and Neijssel OM, 1988. The regulation of the PQQ-linked glucose dehydrogenase in chemostat cultures ofPseudomonas species. Poster abstract, First International Symposium on PQQ and Quinoproteins, Delft.
Hauge, JG, 1964. Glucose dehydrogenase ofBacterium anitratum: an enzyme with a novel prosthetic group. Journal of Biological Chemistry 239: 3630–3639.
Hommes RWJ, 1988. The role of the PQQ-linked glucose dehydrogenase in the physiology ofKlebsiella aerogenes andEscherichia coli. PhD thesis, University of Amsterdam.
Hommes, RWJ, Hell, Bvan, Postma, PW, Neijssel, OM, and Tempest, DW, 1985. The functional significance of glucose dehydrogenase inKlebsiella aerogenes. Archives of Microbiology 143: 163–168.
Hommes, RWJ, Loenen, WAM, Neijssel, OM, and Postma, PW, 1986. Galactose metabolism ingal mutants ofSalmonella typhimurium andEscherichia coli. FEMS Microbiology Letters 36: 187–190.
Hommes, RWJ, Postma, PW, Neijssel, OM, Tempest, DW, Dokter, P, and Duine, JA, 1984. Evidence of a quinoprotein glucose dehydrogenase apoenzyme in several strains ofEscherichia coli. FEMS Microbiology Letters 24: 329–333.
Mackechnie, I, and Dawes, EA, 1969. An evaluation of the pathways of metabolism of glucose, gluconate and 2-oxogluconate byPseudomonas aeruginosa by measurement of molar growth yields. Journal of General Microbiology 55: 341–349.
Matsushita, K, and Ameyama, M, 1982. D-Glucose dehydrogenase fromPseudomonas fluorescens, membrane-bound. Methods in Enzymology 89: 149–154.
Matsushita, K, Nonobe, M, Shinagawa, E, Adachi, O and Ameyama, M, 1987. Reconstitution of pyrroloquinoline quinone-dependent D-glucose oxidase respiratory chain ofEscherichia coli with cytochromeo oxidase. Journal of Bacteriology 169: 205–209.
Mulder, MM, Teixeira de Mattos, MJ, Postma, PW, and Dam, Kvan, 1986. Energetic consequences of multiple K+ uptake systems inEscherichia coli. Biochimica et Biophysica Acta 851: 223–228.
Neijssel, OM, 1977. The effect of 2,4-dinitrophenol on the growth ofKlebsiella aerogenes NCTC 418 in aerobic chemostat cultures. FEMS Microbiology Letters 1: 47–50.
Neijssel, OM, and Tempest, DW, 1975. The regulation of carbohydrate metabolism inKlebsiella aerogenes NCTC 418 organisms, growing in chemostat culture. Archives of Microbiology 106: 251–258.
Neijssel, OM, Tempest, DW, Postma, PW, Duine, JA, and Frank Jzn, J 1983. Glucose metabolism by K+-limitedKlebsiella aerogenes: evidence for the involvement of a quinoprotein glucose dehydrogenase. FEMS Microbiology Letters 20: 35–39.
Nelson, DL, and Kennedy, EP, 1972. Transport of magnesium by a repressible and a nonrepressible system inEscherichia coli. Proceedings of the National Academy of Sciences of the U.S.A. 69: 1091–1093.
Ng, FMW, and Dawes, EA, 1973. Chemostat studies on the regulation of glucose metabolism inPseudomonas aeruginosa by citrate. Biochemical Journal 132: 129–140.
Niederpruem, DJ, and Doudoroff, M, 1965. Cofactor-dependent aldose dehydrogenase fromRhodopseudomonas sphaeroides. Journal of Bacteriology 89: 697–705.
Schie BJ van, 1987. The physiological function of gluconic acid production inAcinetobacter species and other gram-negative bacteria. Implications for energy conservation. PhD thesis, University of Technology, Delft.
Schie, BJvan, Dijken, JPvan, and Kuenen, JG, 1984. Non-coordinated synthesis of glucose dehydrogenase and its prosthetic group PQQ inAcinetobacter andPseudomonas species. FEMS Microbiology Letters 24: 133–138.
Schie, BJvan, Hellingwerf, KJ, Dijken, JPvan, Elferink, MGL, Dijl, JMvan, Kuenen, JG, and Konings, WN, 1985. Energy transduction by electron transfer via a pyrrolo-quinoline quinone-dependent glucose dehydrogenase inEscherichia coli, Pseudomonas aeruginosa, andAcinetobacter calcoaceticus (var.Lwoffi). Journal of Bacteriology 163: 493–499.
Schie, BJvan, Mooy, OHde, Linton, JD, Dijken, JPvan, and Kuenen, JG, 1987a. PQQ-dependent production of gluconic acid byAcinetobacter, Agrobacterium, andRhizobium species. Journal of General Microbiology 133: 867–875.
Schie, BJvan, Pronk, JT, Hellingwerf, KJ, Dijken, JPvan, and Kuenen, JG, 1987b. Glucose-dehydrogenase-mediated solute transport and ATP synthesis inAcinetobacter calcoaceticus. Journal of General Microbiology 133: 3427–3435.
Schie, BJvan, Rouwenhorst, RJ, Bont, JAMde, Dijken, JPvan, and Kuenen, JG, 1987c. An in vivo analysis of the energetics of aldose oxidation byAcinetobacter calcoaceticus. Applied Microbiology and Biotechnology 26: 560–567.
Uspenskaya, SN, and Loitsyanskaya, MS 1979. Effectiveness of the utilization of glucose byGluconobacter oxydans. Microbiology 48: 306–310.
Willsky, GR, and Malamy, MH, 1974. The loss of the phoS periplasmic protein leads to a change in the specificity of a constitutive inorganic phosphate transport system inEscherichia coli. Biochemical and Biophysical Research Communications 60: 226–233.
Willsky, GR, and Malamy, MH, 1976. Control of the synthesis of alkaline phosphatase and the phosphate binding protein inEscherichia coli. Journal of Bacteriology 127: 595–609.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Neijssel, O.M., Hommes, R.W.J., Postma, P.W. et al. Physiological significance and bioenergetic aspects of glucose dehydrogenase. Antonie van Leeuwenhoek 56, 51–61 (1989). https://doi.org/10.1007/BF00822584
Issue Date:
DOI: https://doi.org/10.1007/BF00822584