Summary
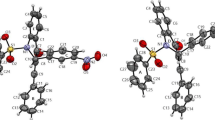
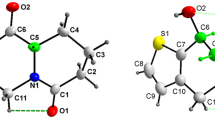
1-Alkylpyrano[3,4-b]indol-3-ones3 react via a Diels-Alder step with an aryne or N-phenylmaleimide to furnish the new [b]annellated carbazoles4–10 in a one-pot process. In an analogous procedure, the in situ generated N-benzoylindole-2,3-quinodimethane (13) reacted with quinones to furnish the dioxocarbazoles14–16. Compounds4–8 and14–16 with a coplanar skeleton are members of a class of potential DNA intercalators, as has been shown for5 and8 by X-ray structural analysis. On the basis of the geometries determined by X-ray crystallography, the intercalative binding of these molecules with a Watson-Crick mini-helix was predicted by molecular modeling methods.
Zusammenfassung
1-Alkylpyrano[3,4-b]indol-3-one3 reagieren über einen Diels-Alder-Schritt mit Arin oder N-Phenylmaleinimid zu [b]annellierten Carbazolen4–10 in einer Einstufenreaktion. In analoger Weise reagiert ein in situ erzeugtes N-Benzoylindol-2,3-chinodimethan13 mit Chinonen zu den Dioxocarbazolen14–16. Die Verbindungen4–8 und14–16 gehören infolge ihrer coplanaren Struktur zur Klasse potentieller DNA-Interkalatoren. Auf der Basis von Röntgenstrukturanalysen von5 und8 wird die interkalative Bindung mit einer Watson-Crick Minihelix durch Molecular Modeling vorhergesagt.
Similar content being viewed by others
References
Albrecht W. L., Fleming R. W., Hogan S. W., Mayer G. D. (1977) J. Med. Chem.20: 364
Tabka T. Robba M. (1988) Eur. J. Med. Chem.23: 119
Kansal V. K., Poitier P. (1986) Tetrahedron42: 2389
Pindur U. (1987) Pharm. Uns. Zeit16: 47
Larue L., Rivalle C., Muzard G., Paoletti C., Bisagni E., Paoletti J. (1988) J. Med. Chem.31: 1951; Auclair C. (1987) Arch. Biochem. Biophys.259: 1259
Lescot E., Muzard G., Markovits J., Belleney, J., Roques B. D., Le Pecq J. B. (1986) J. Med. Chem.29: 1731
Wakeling L. P. G. (1986) Medicin. Res. Rev.6: 275
Kuroda R. (1989) J. Synth. Org. Chem. (Jpn.)47: 547
Pindur U. (1980) Dtsch. Apotheker Ztg.120: 1691
Jain S. C., Bhandary K. K., Sobell H. M. (1979) J. Med. Biol.135: 813
Pindur U., Erfanian-Abdoust H. (1988) Liebigs Ann. Chem.: 803; Pindur U., Eitel M. (1990) J. Org. Chem.55: 5368
Pindur U., Erfanian-Abdoust H. (1988) Chimia42: 180
Pindur U., Erfanian-Abdoust H. (1989) Liebigs Ann. Chem.: 227
Review: Pindur U., Erfanian-Abdoust H. (1989) Chem. Rev.89: 1681
Pindur U., Erfanian-Abdoust H. (1989) Heterocycles29: 1709; Pindur U., Erfanin-Abdoust H. (1990) Liebigs Ann. Chem.: 771; Harber M., Pindur U. (1991) Tetrahedron47: 1925; Pinder U., Pfeuffer L., Eitel M., Rogge M., Haber M. (1991) Monatsh. Chem.122: 291
Plieninger H., Müller W., Weinert K. (1964) Chem. Ber.97: 667
Moody C. J., Shah P. (1989) J. Chem. Soc., Perkin Trans.1: 376; Moody C. J., Shah P., Knowles P. (1988) J. Chem. Soc., Perkin Trans.1: 3249
Van Doren P., Vanderzande D., Toppet S., Hoornaert G. (1989) Tetrahedron45: 6761; Van Doren P., Compernolla P., Hoornaert G. (1990) Tetrahedron46: 4023
Pindur U., Haber M. (1991) Heterocycles32: 1463
Sheldrick G. M. (1986) Programm zur Lösung von Kristallstrukturen, Göttingen
Sheldrick G. M. (1975) Programs for Crystal Structure Determination, Cambridge, Version 8/1976
MMX force field programme from K. E. Gilbert and J. J. Gajewski based on MM2 (Allinger, QCPE 395) and MMP1 (Allinger, QCPE 318) modified by K. Steliou, Serena Software Ltd.; this programme has implemented the Monte Carlo Metropolis algorithms
Kuroda R., Sainsbury M. (1984) J. Chem. Soc., Perkin Trans.1: 1751
Maraun R., Gesh N. (1989) Biopolymers28: 835; Chen K.-X., Gresh N., Pullman B. (1987) Biopolymers26: 831, and references cited therein
Molecular modeling programme ALCHEMY II from Evans & Sutherland, Tripos Assoc., Inc., St. Louis, MO, USA
Molecular modeling programme SYBYL 5.3 from Evans & Sutherland, Tripos Assoc., Inc., St. Louis, MO, USA
Application of molecular mechanics calculations to nucleosides: Burkert U., Allinger N. L. (1982) Molecular Mechanics, ACS Monograph 177, American Chemical Society, Washington, DC
For the first molecular mechanics calculations on dinucleotides with ethidium salts, 9-aminoacridine, and proflavine, see: Nuss M. E., Marsh F. J., Kollman P. A. (1979) J. Am. Chem. Soc.101: 825
For a comprehensive discussion of structural aspects of nucleic acids and for a definition of torsional angles in nucleotides, see: W. Saenger W. (1988) Principles of Nucleic Acid Structure, Springer Verlag, New York. For a recent leading review based on crystallographic studies and structural aspects of nucleic acids, see: Kennard O., Hunter W. N. (1991) Angew. Chem.103: 1280; Angew. Chem. Int. Ed. Engl.30: 1245
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dräger, M., Haber, M., Erfanian-Abdoust, H. et al. New potential DNA intercalators of the carbazole series from indole-2,3-quinodimethanes: Synthesis, crystal structure, and molecular modeling with a watson-crick mini-helix. Monatsh Chem 124, 559–576 (1993). https://doi.org/10.1007/BF00819524
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00819524