Summary
Stereochemistry and tautomerism of hypericin, pseudohypericin, and several of their partial structure models were investigated using an MM2 derived force field method. Besides the “propeller” type conformer, which was also found by the X-ray crystallographic study, the complicated energy hypersurface was shown to contain a novel “double-butterfly” conformer of similar stability. The upper limit interconversion barrier between these conformers and their enantiomers was found to be in the order of 115 kJ/mol.1H-NMR experiments suggested a lower limit interconversion barrier of at least 80 kJ/mol. From the ten tautomers possible in principle, the 7,14-species was derived to be the most stable one by at least 48 kJ/mol.
Zusammenfassung
Die Stereochemie von Hypericin, Pseudohypericin und einigen seiner Partialstrukturmodelle wurde mit Hilfe einer von MM2 abgeleiteten Kraftfeldmethodik untersucht. In der komplizierten Energiehyperfläche wurde neben dem auch durch Röntgenstrukturanalyse gefundenen „Propeller“-Konformeren ein neues „Doppelschmetterling“-Konformer ähnlicher Stabilität aufgefunden. Die obere Grenze für die Interkonversionsbarrieren zwischen diesen Konformeren und ihren Enantiomeren sind in der Größenordnung von 115 kJ/mol. Aus1H-NMR-Experimenten konnte eine untere Grenze von wenigstens 80 kJ/mol abgeleitet werden. Es wurde gefunden, daß von den zehn prinzipiell möglichen Tautomeren die 7,14-Spezies die um wenigstens 48 kJ/mol stabilste ist.
Similar content being viewed by others
References
Brockmann H., Haschad M. N., Maier K., Phol F. (1939) Naturwiss.27: 550, Knox J. P., Dodge A. D. (1985) Plant Cell Environment8: 19; Roth L. (1990) Hypericum-Hypericin, Botanik Inhaltsstoffe Wirkung; ecomed Verlagsgesellschaft mbH, Landsberg
Song P.-S. (1989) Molecular Electronics, Biosensors, and Biocomputers. Plenum Press, New York; Walker E. B., Lee T. Y., Song P.-S. (1979) Biochim. Biophys. Acta587: 129; Song P.-S. (1981) Biochim. Biophys. Acta639: 1
De Riccardis F., Iorizzi M., Minale L., Riccio R., Richer de Froges R., Debitus C. (1991) J. Org. Chem.56: 6781
Meruelo D., Lavie G., Lavie D. (1988) Proc. Natl. Acad. Sci. USA85: 5230; Lavie G., Valentine F., Levin B., Mazur Y., Gallo G., Lavie D., Wiener D., Meruelo D. (1989) Proc. Natl. Acad. Sci. USA86: 5963; Tang J., Colacino J. M., Larsen S. H., Spitzer W. (1990) Antiviral Research13: 313; Lavie D., Revel M., Rotmann D., Van de Velde V. (1988) European Patent Appl. 0 256 452, A2; Lopez-Bazzocchi I., Hudson J. B., Towers G. H. N. (1991) Photochem. Photobiol.54: 95
Falk H., Schoppel G. (1991) Monatsh. Chem.122: 739; Falk H., Meyer J., Oberreiter M. (1992) Monatsh. Chem.123: 277; Falk H., Schoppel G. (1992) Monatsh. Chem.123: 931; Falk H., Schmitzberger W. (1992) Monatsh. Chem.123: 731
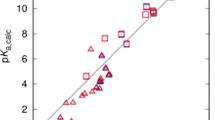
Etzlstorfer C., Falk H., Müller N. (1993) Monatsh. Chem.124: 431
Sprague J. T., Tai J. C., Yuh Y., Allinger N. L. (1987) J. Comp. Chem.8: 581; Liljefors T., Tai J. C., Yuh Y., Allinger N. L. (1987) J. Comp. Chem.8: 1051
Ball & Stick 3.5: Müller N., Falk A. (1993) Cherwell Scientific Publ. Ltd., Oxford, U.K.
Falk H., Schmitzberger W. (1992) Monatsh. Chem.123: 731
Sheldrick G. M. (1986) SHELX-86, A Computer Program for Crystal Structure Solution. Univ. of Göttingen, BRD; Sheldrick G. M. (1976) SHELX-76, A Computer Program for Crystal Structure Determination. Univ. of Cambridge, UK; Walker N., Stuart D. (1983) Acta Crystallogr.A39: 158, DIFABS
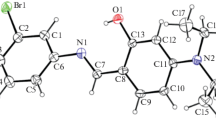
Camerman A., Trotter J. (1964) Proc. Roy. Soc. LondonA279: 129
Hirsch J. A. (1967) Topics in Stereochem.1: 199
Thomson R. H. (1987) Naturally Occurring Quinones III. Chapman and Hall, London
Brockmann H., Spitzner D. (1975) Tetrahedron Lett.1975: 37
Randic M. (1975) Tetrahedron31: 1477
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Etzlstorfer, C., Falk, H., Müller, N. et al. Tautomerism and stereochemistry of hypericin: Force field, NMR, and X-ray crystallographic investigations. Monatsh Chem 124, 751–761 (1993). https://doi.org/10.1007/BF00817311
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00817311