Summary
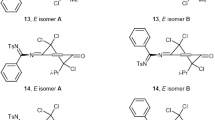
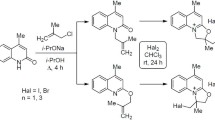
3-Substituted 4-hydroxy-2(1H)-quinolones3,5,7 are halogenated with bromine or sulfuryl chloride to yield the quinolinediones9 or10. Reaction of3,5,7 with chloroform gives the dichloromethyl quinolinediones11. Halogen exchange leads from the chloro quinolinediones10 to fluoro quinolinedones12 and to azido quinolinediones13. Similarly the dichloro quinolinedione10 an reacts to the difluoro quinolinedione14, which is reduced to the 3-fluoro-4-hydroxyquinolone16 and reacts again with sulfuryl chloride to give the mixed 3-chloro-3-fluoroquinolinedione15.
Zusammenfassung
3-Substituierte 4-Hydroxy-2-chinolone3,5,7 reagieren mit elementarem Brom oder Sulfurylchlorid zu den 3-Halogen-chinolindionen9 oder10. Mit Chloroform reagieren die Hydroxychinolone3,5,7 zu den 3-Dichlormethylchinolondionen11. Halogenaustausch an10 führt zu den 3-Fluorchinolindionen12 und zu 3-Azidochinolindionen13. Ähnlich reagiert 3,3-Dichlorochinolindion10 an zu 3,3-Fluorchinolindion14, das zum 3-Fluor-4-hydroxychinolon16 reduziert werden kann und in weiterer Folge mit Sulfurylchlorid zum gemischten 3-Chlor-3-fluor-chinolindion15 reagiert.
Similar content being viewed by others
References
Organic Azides in Heterocyclic Synthesis, part 16. Part 15: Roschger P., Fiala W., Stadlbauer W., J. Heterocycl. Chem. (1982) in press
Kappe C. O., unpublished
Malle E., Stadlbauer W., Ostermann G., Hofmann B., Leis H. J., Kostner G. M. (1990) Eur. J. Med. Chem.25: 137
Laschober R., Stadlbauer W. (1990) Liebigs Ann. Chem.1990: 1083
Kitamura S., Hashizume K., Iida T., Miyashitsa E., Shirata K., Kase H. (1986) J. Antibiot.39: 1160
Neuenhaus W., Budzikiewicz H., Korth H., Pulverer G. (1979) Z. Naturforsch.34 b: 313; Budzikiewicz H., Schaller U., Korth H., Pulverer G. (1979) Monatsh. Chem.110: 974
Ziegler E., Salvador R., Kappe Th. (1962) Monatsh. Chem.93: 1376
Ziegler E., Kappe Th. (1963) Monatsh. Chem.94: 447
Fournier C., Decombe J. (1967) Bull. Soc. Chim. Fr.1967: 3367; (1967) C. R. Acad. Sci. Paris, Ser. C.265: 1169
Witoszynsky Th. (1972) Ph. D. thesis. University of Graz, p. 58; Lakhvich F. A., Kozinets V. A., Rubinov D. B., Akhrem A. A. (1987) Zh. Org. Khim.23: 2626
Ziegler E., Salvador R., Kappe Th. (1963) Monatsh. Chem.94: 941
Purrington S. T., Bumgardner C. L., Lazaridis N. V., Singh P. (1987) J. Org. Chem.52: 4307
Visser G. W. M., Herder R. E., De Kanter F. J. J., Herscheid J. D. M. (1988) J. Chem. Soc. Perkin Trans. 11988: 1203
Bohlmann R. (1990) Nachr. Chem. Techn. Lab.38: 40
Rieux C., Langlois B., Gallo R. (1990) C. R. Acad. Sci., Ser. II310: 25; Cox D. P., Terpinsky J., Lawrynowicz W. (1984) J. Org. Chem.49: 3216
Liotta C. L., Harris H. P. (1974) J. Am. Chem. Soc.96: 2251
Zima V., Pytela O., Kavalek J., Vecera M. (1989) Coll. Czech. Chem. Commun.54: 2715
Stadlbauer W., Schmut O., Kappe Th. (1980) Monatsh. Chem.111: 1005; Baumgarten P., Kärgel W. (1927) Ber. Dtsch. Chem. Ges.60: 832
Kappe Th., Karem A. S., Stadlbauer W. (1987) J. Heterocyclic Chem.25: 857
Aldrich Chemie GmbH, Steinheim, FRG, catalog no. 30, 759-9
Asahina Y., Inubuse M. (1932) Ber. Dtsch. Chem. Ges.65: 61
Krauch H., Kunz W. (1976) Reaktionen der Organischen Chemie, Alfred Hüthig Verlag, Heidelberg, p. 613
Kappe Th., Ziegler E. (1969) Synthesis: 74
Kappe Th., Fritz P. F., Ziegler E. (1973) Chem. Ber.106: 1927
Stadlbauer W., Kappe Th. (1982) Z. Naturforsch.37 b: 1196, and references cited therein
Stadlbauer W., Kappe Th. (1985) Monatsh. Chem.116: 1005
Lang G. (1972) Ph. D. thesis, Karl-Franzens University of Graz, p. 84–85
Author information
Authors and Affiliations
Additional information
Herrn Prof. Dr. Erich Ziegler in freundschaftlicher Verbundenheit zum 80. Geburtstag gewidmet.
Rights and permissions
About this article
Cite this article
Stadlbauer, W., Laschober, R., Lutschouig, H. et al. Halogenation reactions in position 3 of quinoline-2,4-dione systems by electrophilic substitution and halogen exchange. Monatsh Chem 123, 617–636 (1992). https://doi.org/10.1007/BF00816857
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00816857