Summary
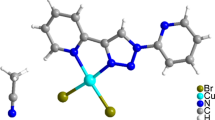
New dicyanamide complexes of the typeM[N(CN)2]2 L 2 (M=Cu or Ni;L=2-, 3-, 4-aminopyridine, 2-amino-5-nitropyridine) and Co[N(CN)2]2 (2-amino-5-nitropyridine)2 were prepared and studied by spectroscopic methods as well as by room-temperature magnetic moments. The results show that the Cu(II) complexes have elongated pseudooctahedral structures while the Ni(II) and Co(II) complexes are octahedral. In most cases the N(CN)2 groups are in bridging function and through their cyanide nitrogens, or-more rarely-amide and cyanide nitrogens connect the basic structure units into polymeric conglomerates. In the Cu(II) systems exchange coupling is seen from the μeff value or ESR spectrum.
Similar content being viewed by others
References
Kohout J., Hvastijová M., Gažo J. (1978) Coord. Chem. Rev.27: 141; Hvastijová M., Kohout J., Gažo J. (1981) Monatsh. Chem.112: 1143; Kohout J., Hvastijová M., Gažo J., Nádvorník M. (1979) Inorg. Chim. Acta37: 225 (1979)
Kohout J., Hvastijová M., Köhler H., Omelka L. (1990) Collect. Czechoslov. Chem. Commun.55: 435
Kohout J., Hvastijová M., Omelka L. (1985) Transition Met. Chem. (Weinheim)10: 155
Hvastijová M., Kohout J., Wusterhausen H., Köhler H. (1984) Anorg. Allg. Chem.510: 37
Golub A. M., Köhler H., Skopenko V. V. (eds.) (1986) Chemistry of Pseudohalides, Elsevier Amsterdam
Kohout J., Hvastijová M., Mašlejová A., Gažo J., Omelka L. (1977) Anorg. Allg. Chem.434: 29
Nakamoto K. (1978) Infrared and Raman Spectra of Inorganic and Coordination Compounds, 2nd Ed. Wiley-Interscience, New York; Reedijk J. (1979) Transition Met. Chem. (Weinheim)4: 335
Mason S. F. J. Chem. Soc.1958: 3619; Katritzky A. R., Jones R. A. J. Chem. Soc.1959: 3674
Valach F., Dunaj-Jurčo M. (1982) Acta Crystallogr.B 38: 2145
Hvastijová M., Kohout J., Köhler H., Ondrejovič G. (1988) Anorg. Allg. Chem.566: 111
Hathaway B. J., Billing D. E. (1970) Coord. Chem. Rev.5: 143
Perrin D. D. (1965) Dissociation Constants of Organic Bases in Aqueous Solution. Butterworths, London
Lever A. B. P. (1984) Inorganic Electronic Spectroscopy. Elsevier, Amsterdam
Jörgensen C. K. (1962) Absorption Spectra and Chemical Bonding in Complexes. Pergamon Press, Oxford
Mroziński J., Kohout J., Hvastijová M., Köhler H. (1986) Transition Met. Chem. (Weinheim)11: 481; Kohout J., Mroziński J., Hvastijová M. (1989) Phys. Chem. (Leipzig)270: 975
Mroziński J., Kohout J., Hvastijová M.: Publikation in Vorbereitung
Earnshaw A. (1968) Introduction to Magnetochemistry: Academic Press, London
Hathaway B., Duggan M., Murphy A., Mullane J., Power Ch., Walsh A., Walsh B. (1981) Coord. Chem. Rev.36: 267
Plesch G., Friebel C., Švajlenová O., Krätsmár-Šmogrovič J. (1987) Inorg. Chim. Acta129: 81; Sivý J., Kettmann V., Krätsmár-Šmogrovič J., Švajlenová O., Friebel C., Plesch G. (1990) Anorg. Allg. Chem.583: 55
Hitchman M. A. J. Chem. Soc.A 1970: 4
Author information
Authors and Affiliations
Additional information
Professor Viktor Gutmann zum 70. Geburtstag gewidmet
Rights and permissions
About this article
Cite this article
Hvastijová, M., Kohout, J. & Köhler, H. Strukturelle Charakterisierung der Dicyanamid-Komplexe von Kupfer(II), Nickel(II) bzw. Kobalt(II) mit Aminopyridinen. Monatsh Chem 123, 493–500 (1992). https://doi.org/10.1007/BF00816842
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00816842