Abstract
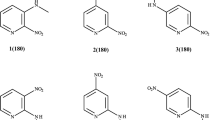
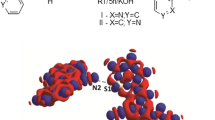
The crystal structures of pyrene and substituted and complexed derivatives of pyrene have been investigated by X-ray and neutron diffraction. The geometry of the pyrene skeleton has been determined experimentally with high accuracy and calculated by quantum chemical methods. In the cases reported in the literature and cited here the pyrene skeleton has the molecular symmetry mmm or mm2 with values for the bond lengths of the six symmetrically independent bondsa, b, c, d, e, f differing significantly in the limits of error. Mean values of a number of experimental and theoretical bond lengths are given and can be considered as standard values for the mm2 symmetric pyrene skeleton. In the case of substitution of the pyrene in 3-position with a polar heterocyclic molecule of the azomethine-imine type the mm2 symmetry vanishes, a C–H ... N intramolecular hydrogen bond arises and the directly neighbouring pyrene units are not packed parallel with their planes to each other, but they are considerably tilted. Relatively narrow intermolekular C-C contacts, 3.314 and 3.368 Å, have been observed. The conclusion is drawn that the asymmetry of the pyrene molecule and a tilt of directly neighbouring pyrene units in the crystal packing can be induced by substitution e. g. with suitable polar heterocycles.
Similar content being viewed by others
Literatur
Robertson J. M., White J. G., J. Chem. Soc.61, 358 (1947).
Kitaigorodski A. I., Molekülkristalle, S. 43. Berlin: Akademie Verlag (DDR). 1970.
Camerman A., Trotter J., Acta Cryst.18, 636 (1965).
Allmann R., Z. Krist.132, 129 (1970).
Kai Y., Hama F., Yasuoka N., Kasai N., Acta Cryst.B 34, 1263 (1978).
Hazell A. C., Larsen F. K., Lehmann M. S., Acta Cryst.B 28, 2977 (1972).
Ikamoto I., Kuroda H., Acta Cryst.B 24, 383 (1968).
Krebs-Larsen F., Little R. G., Coppens P., Acta Cryst.B 31, 430 (1975).
Hazell A. C., Lomborg J. G., Acta Cryst.B 28, 1059 (1972).
Herbstein F. H., Snyman J. A., Phil. Trans. Roy. Soc.A 264, 235 (1969).
Bernstein J., Regev H., Herbstein F. H., Main P., Rizvi S. H., Sasvari K., Turcsanyi B., Proc. Roy. Soc.A 347, 419 (1975).
Irngartinger H.,Kirrstetter R. G. H.,Krieger C.,Rodenwald H.,Staab H. A., Tetrahedron Lett.1977, 1425.
Doherty R. M., Steward J. M., Acta Cryst.B 38, 859 (1982).
Barnes J. C.,Chudek J. A.,Foster R.,Jarrett F.,Mackie F.,Paton J.,Twiselton D. R., Tetrahedron1981, 1595.
Moffit W. E., Coulson C. A., Proc. Roy. Soc. (London)60, 309 (1948).
Warren K. D., Yandle J. R., Theoret. Chim. Acta12, 267 (1968).
Pauling L., Acta Cryst.B 36, 1898 (1980).
Kulpe S., Seidel I., Geissler G., Cryst. Res. & Technol.18, 339 (1983).
Dorn H., Otto A., Chem. Ber.101, 3287 (1968).
Kulpe S., Z. Chem.20, 377 (1980).
Kulpe S., Z. Chem.5, 184 (1985).
Dähne S., Kulpe S., Structural Principles of Unsaturated Organic Compounds, Abhandlg. d. Akad. Wiss. DDR, N8. Berlin: Akademie Verlag (DDR). 1977.
Mehlhorn A., Fabian J., Kulpe S., J. prakt. Chem.326, 303 (1984).
Radeglia R., Dorn H., Z. Chem.22, 313 (1982).
Kulpe S., Seidel I., Z. phys. Chem. (Leipzig)264, 25 (1983).
Motherwell S., ENY. A Program for the Calculation of Potential Energies in Molecular Structures, Univ. Chemical Laboratory Cambridge, England (1973).
Kimura M., Kashino S., Morosawa S., Haisa M., Acta Cryst.C 40, 1612 (1984);Kimura M., Nukada K., Satake K., Morosawa S., Tamagake K., J. Chem. Soc. Perkin Trans. 1, in press.
Author information
Authors and Affiliations
Additional information
Juli 1985.
Rights and permissions
About this article
Cite this article
Kulpe, S., Seidel, I. Das Pyren und der Einfluß von Substitution oder Komplexie-rung auf seine Geometrie und Packung im kristallinen Zustand. Monatsh Chem 117, 295–304 (1986). https://doi.org/10.1007/BF00816523
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00816523