Conclusions
-
1.
The structure of oxide films and the resistance of pearlitic steel to scaling at 600°C in ambient air atmosphere depends on the degree of alloying.
-
2.
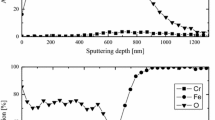
An increase in the concentration of the alloying elements causes the oxidation resistance of steel in the air medium to rise at 600°C. The best results were obtained for steel with 7% Cr: the magnitude of weight increase is 0.3 mg/cm2 after 1000-hours oxidizing heating at 600°C. A single-phase oxide film (oxide of the α-Fe2O3 typed) was observable on the surface of the metal.
The lowest increase in oxidation resistance was registered for steel alloyed with cobalt. After prolonged oxidizing, along with α-Fe2O3 and Fe3O4, FeO wustite was also revealed in the oxide film of steels alloyed by cobalt.
-
3.
It is recommended to conduct electron diffraction studies at the early stages of the steel oxidizing process at 300–600°C (with holding times amounting to 1 hour). Additional alloying of pearlitic steel with chrome, silicon, aluminum, cobalt, tungsten, and boron raises the temperature at which the second phase (Fe3O4) appears in the oxide film.
A rise of the above temperature depends on the type and the concentration of the alloying element.
The steels for which the highest temperature was observed during the transition of the oxide film from a single- to two-phase state possess the highest resistance to scaling at 600°C.
Similar content being viewed by others
References
V.I. Arkharov. Oxidation of Metals at High Temperature. Metallurgizdat, 1945.
A.P. Gulyayev. Metallography, Oborongiz, 1951.
O. Kubashevskiy, and Hopkins, B. Oxidation of Metals and Alloys, Foreign Literature, 1955.
L.B. Pfeifl. Journal of the Iron and Steel Institute, 110, 501, 1929; 123, 237, 1931.
V.V. Ipat'yev. Sibirskaya, V.V., Taubina, M.G., Red'ko, Yu.D., Tikhomirov, V.I., Orlova, G.M., and Morozova, M. P. Scientific Papers of the Leningrad State University, No. 175, Series of Chemical Sciences, No. 14, 88–116, and 128–167, 1954. Ibid, No. 227-Series of Chemical Sciences, No. 17, 5–45, 1957.
E. Sheil, and Kiwit. Archive der Eisenhuttenwesen, Vol.8, 1936.
V.V. Ipat'yev, and Orlova, G.M. Journal of Applied Chemistry, Vol. 29, 6, 1956.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yuganova, S.A., Sorokina, Y.G. The structure of oxide films and scaling resistance of pearlite steel, and their dependence on alloying. Met Sci Heat Treat 3, 279–282 (1961). https://doi.org/10.1007/BF00813010
Issue Date:
DOI: https://doi.org/10.1007/BF00813010