Summary
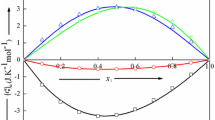
Excess molar volumesV E and excess molar heat capacitiesC EP at constant pressure have been determined, as a function of mole fractionx 1 at 298.15 K and atmospheric pressure, for the two liquid mixtures {pyridine or piperidine+cyclohexane}. The instruments used were a vibrating-tube densimeter and a Picker flow microcalorimeter, respectively. The two systems show positive excess volumes withV E(x 1=0.5)=0.531 cm3·mol−1 for {pyridine+cyclohexane} and 0.295 cm3·mol−1 for {piperidine+cyclohexane}. The curveC EP vs. x 1 for {pyridine+cyclohexane} shows a rather complex S-shape:C EP is negative at small mole fractionsx 1 of pyridine and positive forx 1>0.22, roughly.C EP of the piperidine system is negative throughout and strongly asymmetric with the minimumC EP (x 1,min)=−2.32J·K−1·mol−1 being situated at a mole fraction of piperidinex 1,min≈0.27.
Zusammenfassung
Für die beiden flüssigen Mischungen {Pyridin oder Piperidin+Cyclohexan} wurden molare ZusatzvoluminaV E und molare ZusatzwärmekapazitätenC EP bei konstantem Druck als Funktion des Molenbruchsx 1 bei 298.15K bestimmt. Die Messungen wurden mit einem Biegeschwinger-Dichtemeßgerät bzw. einem Strömungsmikrokalorimeter nach Picker durchgeführt. Die Zusatzmolvolumina beider Systeme sind positiv mitV E(x 1=0.5)=0.531 cm3·mol−1 für {Pyridin+Cyclohexan} und 0.295 cm3·mol−1 für {Piperidin+Cyclohexan}. Die KurveC EP vs. x 1 des Systems {Pyridin+Cyclohexan} zeigt einen ungewöhnlichen S-förmigen Verlauf: bei kleinen Molenbrüchenx 1 von Pyridin istC EP negativ, fürx 1>0.22 istC EP positiv. Die molare Zusatzwärmekapazität des Piperidinsystems ist überall negativ und stark unsymmetrisch: im Minimum beix 1,min≈0.27 findet manC EP (x 1,min)=−2.32J·K−1·mol−1.
Similar content being viewed by others
References
Wilhelm E. (1985) Thermochim. Acta.94: 47
Wilhelm E. (1990) Thermochim. Acta162: 43
Grolier, J.-P. E., Wilhelm E. (1991) Pure Appl. Chem.63: 1427
Inglese A., Wilhelm E. Grolier J.-P. E. 37th Annual Calorimetry Conference, Snowbird, Utah, USA, 20–23 July 1982, Paper No. 54;
Grolier J.-P. E., Inglese A., Wilhelm E. (1984) J. Chem. Thermodyn.16: 67
Wilhelm E., Inglese A., Roux A. H., Grolier J.-P. E. (1984) Calorim. Anal. Therm.15: 108
Roux A. H., Grolier J.-P. E., Inglese A., Wilhelm E. (1984) Ber. Bunsenges. Phys. Chem.88: 986
Lainez A., Roux-Desgranges G., Grolier J.-P.E., Wilhelm, E. (1985) Fluid Phase Equil.20: 47
Lainez A., Rodrigo M., Roux A. H., Grolier J.-P.E., Wilhelm E. (1985) Calorim. Anal.16: 153
Lainez E., Wilhelm E., Roux-Desgranges G., Grolier J.-P. E. (1985) J. Chem. Thermodyn.17: 1153
Wilhelm E., Roux A. H., Roux-Desgranges G., Rodrigo M., Lainez A., Grolier J.-P. E. (1986) Calorim. Anal. Therm.17: 12
Wilhelm E., Lainez A., Rodrigo M., Roux A. H., Grolier J.-P. E. (1988) Calorim. Anal. Therm.19: C20.1
Wilhelm E., Lainez A., Grolier J.-P. E. (1988) Fluid Phase Equil.49: 233
Wilhelm E., Jimenez E., Roux-Desgranges G., Grolier J.-P. E. (1991) Solution Chem.20: 17
Grolier J.-P. E., Roux-Desgranges G., Berkane M., Wilhelm E. (1991) J. Chem. Thermodyn.23: 421
Lainez A., Rodrigo M. M., Wilhelm E., Grolier J.-P. E (1992) J. Solution Chem.21: 49
Grolier J.-P. E., Roux-Desgranges G., Berkane M., Jimenez E., Wilhelm E. (1993) J. Chem. Thermodyn.25: 41
Grolier J.-P. E., Roux-Desgranges G., Berkane M., Wilhelm E. (1994) J. Solution Chem.23: 153
IUPAC (1992) Pure Appl. Chem.64: 1519
Grolier J.-P. E., Wilhelm E., Hamedi H. M. (1978) Ber. Bunsenges. Phys. Chem.82: 1282
Kell G. S. (1975) J. Chem. Eng. Data20: 97
Fortier J.-L., Benson G. C. (1976) J. Chem. Thermodyn.8: 411
Wilhelm E., Grolier J.-P. E., Karbalai Ghassemi M. H. (1977) Ber. Bunsenges. Phys. Chem.81: 925
Fortier J.-L., Benson G. C., Picker P. (1976) J. Chem. Thermodyn.88: 289
Riddick J. A., Bunger W. B., Sakano T. K. (1986) Organic Solvents, 4th ed. Wiley, New York
Lam V. T., Picker P., Patterson D., Tancrede P. (1974) J. Chem. Soc., Faraday Trans.II 70: 1465
Nakanishi K., Shirai H., Nakasato K. (1968) J. Chem. Eng. Data13: 188
Nakanishi K., Wada H., Touhara H. (1975) J. Chem. Thermodyn.7: 1125
Moelwyn-Hughes E. A., Thorpe P. L. (1964) Proc. Roy. Soc. (London) A278: 574
Timmermans J. (1965) Physico-Chemical Constants of Pure Organic Compounds. Elsevier, New York
Tanaka R. (1982) J. Chem. Thermodyn.14: 259
Jimenez E., Roux-Desgranges G., Grolier J.-P. E., Wilhelm E. (1989) Thermochim. Acta151: 99
TRC Thermodynamic Tables (1986) Non Hydrocarbons, p. 9410. Texas A&M University, College Station, Texas, USA
Messerly J. F., Todd S. S., Finke H. L., Good W. D., Gammon B. E. (1988) J. Chem. Thermodyn.20: 209
Woycicki W., Sadowska K. W. (1968) Bull. Acad. Pol. Sci., Ser. Sci. Chim.16: 147
Brzostowski W., Brun B., Salvinien J. (1969) J. Chim. Phys.66: 313
Kowalski B., Boniecka A., Orszagh A. (1978) Polish J. Chem.52: 1079
Weclawski J. (1983) Fluid Phase Equil.12: 155
Woycicki W., Sadowska K. W. (1968) Bull. Acad. Pol. Sci., Ser. Sci. Chim.16: 365
Roveillo J., Gomel M. (1968) C. R. Acad. Sci. (Paris), Ser. C266: 845
Murakami T., Murakami S., Fujishiro R. (1969) Bull. Chem. Soc. Japan42: 35 (1969)
Malanowski S., Patz R., Rätzsch M. T., Wolfarth C. (1979) Fluid Phase Equil.3: 291
Konakbaeva E. G. (1985) Int. DATA Ser., Sel. Data Mixtures, Ser. A: 51
Singh P. P., Sharma S. P. (1985) Thermochim. Acta88: 467
Rowlinson J. S., Swinton F. L. (1982) Liquids and Liquid Mixtures, 3rd ed. McGraw-Hill, New York
Saint-Victor M.-E., Patterson D. (1987) Fluid Phase Equil.35: 237
Guggenheim E. A. (1952) Mixtures. Clarendon Press, Oxford
Kalali H., Kohler F., Svejda P. (1985) Fluid Phase Equil.20: 75
Smets R., Huyskens P. (1978) Bull. Soc. Chim. France, Partie I: 173
Diez D., Ruiz B., Royo R. M., Gutierrez Losa C. (1985) J. Chem. Thermodyn.17: 371
Cabani S., Ceccanti N. (1973) J. Chem. Thermodyn.5: 9
Moelwyn-Hughes E. A., Thorpe, P. L. (1964) Proc. Roy. Soc. (London)A 277: 423
Marcus Y. (1992) J. Solution Chem.21: 1217
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lainez, A., Wilhelm, E. & Grolier, J.P.E. Thermodynamics of liquid mixtures containing hydrocarbons and strongly polar substances:V E andC EP of {pyridine or piperidine+cyclohexane} at 298.15 K. Monatsh Chem 125, 877–885 (1994). https://doi.org/10.1007/BF00812701
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00812701