Summary
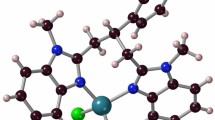
The title complex of copper(II) chloride with picolinic acid was prepared and characterized by spectroscopic and X-ray crystallographic methods. The complex, Cu(C5H4NCOO)Cl, crystallizes tetragonal, space group P42/n (No. 86),a=976.4(1),c=1499.6(4) pm,N=8;R w=0.048 for 543 observed MoKα diffractometer data. In the structure of the complex two μ-chloro bridges form only slightly bent Cu2Cl2 rings [Cu-Cl=224.2(4) and 275.6(4) pm] with Cu...Cu separation of 359.4(2) pm and Cl...Cl separation of 348.7(5) pm. These edge-sharing copper coordination polyhedra are further linked via the N and O donor atoms of the picolinato anions at Cu-N distances of 199.6(12) pm and Cu-O bond lengths of 195.7(8) and 200.6(10) pm, to form a two-dimensional layer structure in which these layers are arranged along theab plane. Each picolinate anion functions as a tetra-dentate ligand: N(1) and O(2) are coordinated to the same Cu(II) center whereas O(1) is bonded to a neighbouring Cu(II) center. O(2) is further bonded to the latter Cu(II) center at a long Cu-O distance of 256.5(8) pm. The electronic, infrared and Raman spectra of the solid complex are reported and discussed.
Zusammenfassung
Der Titelkomplex aus Kupfer(II)chlorid und Picolinsäure wurde dargestellt und mit spektroskopischen und Röntgen-Einkristall-Methoden charakterisiert. Cu(C5H4NCOO)Cl kristallisiert tetragonal, Raumgruppe P42/n (No. 86),a=976.4(1),c=1499.6(4) pm,N=8;R w=0.048 für 543 beobachtete Mokα-Diffraktometerdaten. In der Kristallstruktur bilden zwei μ-chloro-Brücken nur wenig gewinkelte Cu2Cl2-Ringe aus [Cu-Cl=224.2(4) und 275.6(4) pm], mit Cu...Cu Abständen von 359.4(2) pm und Cl...Cl Abständen von 348.7(5) pm. Die kantenverknüpften Koordinationspolyeder der Kupferatome sind in Richtung derab-Ebene über die N- und O-Donoratome der Picolinat-Anionen [mit Cu-N Abständen von 199.6(12) pm und Cu-O Abständen von 195.7(8) und 200.6(10) pm] zu einer zweidimensionalen Schichtstruktur verknüpft. Jedes Picolinat-Anion fungiert als vierzähniger Ligand: N(1) und O(2) sind zum selben Cu(II)-Zentrum gebunden; O(1) ist zum benachbarten Cu(II)-Zentrum koordiniert, zu dem O(2) einen langen Cu-O-Abstand von 256.5(8) pm ausbildet. Die elektronischen, Infrarot- und Raman-Spektren des Festkörper-Komplexes werden berichtet und diskutiert.
Similar content being viewed by others
References
Bovikin B. A., Omel'chenko A. M., Sharonina R. N., Zanina I. A. (1985) Abstracts of Proceedings, 15th. All-Union Chugaeveskii Conference on the Chemistry of Complex Compounds, Vol. 1, p. 229
Kleinstein A., Webb G. A. (1971) J. Inorg. Nucl. Chem.33: 405
Fitzsimmons B. W., Kleinstein A., Seeley N. J., Webb G. A. (1971) Roum. Chem.16: 1197
Anagnostopoulos A., Matthews R. A., Walton R. A. (1972) Can. J. Chem.50: 1307
Fowles G. W. A., Matthews R. A., Walton R. A. (1968) J. Chem. Soc. (A) 1108
Deloume P. J.-P., Loiseleur H., Thomas G. (1973) Acta Cryst.B29: 668
Deloume P. J.-P., Loiseleur H. (1974) Acta Cryst.B30: 607
Chang S. C., Ma J. K. H., Wang J. S., Li N. C. (1972) J. Coord. Chem.2: 31
Takenaka A., Utsumi N., Yamamoto T., Nita I. (1970) Nippon Kagaku Zsashi91: 928
Ellis V. M., Vagg R. S., Watton E. C. (1974) J. Inorg. Nucl. Chem.36: 1031
Ellis V. M., Vagg R. S., Watton E. C. (1974) Aust. J. Chem.27: 1191
Hertzer C. A., Walton R. A. (1975) Inorg. Nucl. Chem. Lett.11: 475
Goher M. A. S., Abdou A. E. H., Mak T. C. W., Inorg. Chim. Acta (submitted)
Goher M. A. S., Abu-Youssef M. A. M., Mautner F. A., Popitsch A. (1992) Polyhedron11: 2137
Walker N., Stuart D. (1983) Acta Cryst.A39: 158
Sheldrick G. M. (1976) SHELX-76, a program for crystal structure determination, University Chemical Laboratory, Cambridge
Sheldrick G. M. (1986) SHELXS-86, Universität Göttingen, F.R.G.
Spek A. L. (1982) In: Sayre D. (ed.) Computational crystallography. Clarendon Press, Oxford, p. 528
Stuart J. M. (1976) THE X-RAY SYSTEM, version of 1976, Technical Report TR-466. University of Maryland, College Park, USA
Ibers J. A., Hamilton W. C. (eds.) (1974) International tables for X-ray crystallography, vol. IV. Kynoch Press, Birmingham, pp. 99 and 149
Additional material to the structure determination can be ordered from Fachinformationszentrum Karlsruhe GmbH, D-7514 Eggenstein-Leopoldshaften 2, referring to the deposition number CSD 57080, the names of the authors and the citation of the paper.
Aliev Z. G., Atovmyan L. O., Sartovskikh E. A., Krinichnii V. I., Kartsev V. G. (1988) Izv. Akad. Nauk SSSR, Sea. Khim.,11: 2495
Megnamisi-Belombe M., Singh P., Boldter D. E., Hatfield W. E., (1984) Inorg. Chem.23: 2578
Yoshida S., Asai M. (1959) Chem. Pharm. Bull. (Japan)7: 162
Nakagawa I., Shimanouchi T. (1964) Spectrochim. Acta20: 429
Lever A. B. P., Lewis J., Nyholm R. S. (1962) J. Chem. Soc. 5262
Goldstein M., Unsworth W. D. (1970) Inorg. Nucl. Chem. Lett.6: 25
Goldstein M., Unsworth W. D. (1972) Spectrochim. Acta28A: 1297
Aggaral R. C., Singh N. K., Singh R. P. (1979) Inorg. Chim. Acta32: L87
Estes W. E., Gavel D. P., Hatfield W. E., Hodgson D. J. (1978) Inorg. Chem.17: 1415
Tomlinson A. A. G., Hathaway B. J. (1968) J. Chem. Soc., (A) 1685; ibid. (1968) 1905.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Goher, M.A.S., Hafez, A.K., Abu-Youssef, M.A.M. et al. Synthesis and spectral and structural characterization of a bridging chloropicolinatocopper(II) complex, Cu(C5H4NCOO)Cl. Monatsh Chem 125, 833–840 (1994). https://doi.org/10.1007/BF00812696
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00812696