Summary
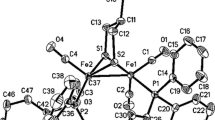
6,6,8,8-Tetramethyl-7-oxa-6,8-disila[3]ferrocenophane2 was obtained from the di(alkoxysilyl) ferrocene (H4C5SiMe2OR)2Fe (R=CH2CH2OCH2CH2OCH2CH2OMe) by hydrolysis and subsequent intramolecular disiloxane formation. 2,2′,3,3′,4,4′,5,5′,6,6,8,8-Dodecamethyl-7-oxa-6,8-disila-[3]ferrocenophane3 was formed by air oxidation of 2,2′3,3′,4,4′,5,5′,6,6,7,7-dodecamethyl-6,7-disila[2]ferrocenophane. The crystal structures of both compounds were determined by single-crystal X-ray diffraction (2:a=8.5330(10),b=15.610(3),c=18.774(5)Å, α=70.68(2), β=77.94(2), γ=75.150(10)°,V=2259.8(8)Å3,Z=6, space group P\(\bar 1\),R=0.045,R w =0.044;3:a=12.388(3),b=9.924(3),c=19.136(10)Å, β=105.11(3)°,V=2271.2(15)Å3,Z=4, space group P21/c,R=0.076,R w =0.060). Owing to the flexibility of the disiloxane bridge,2 and3 are unstrained molecules.
Zusammenfassung
6,6,8,8-Tetramethyl-7-oxa-6,8-disila[3]-ferrocenophan2 entsteht aus dem Di(alkoxysilyl)ferrocen (H4C5SiMe2OR)2Fe (R=CH2CH2OCH2CH2OCH2CH2OMe) durch Hydrolyse und anschließende intramolekulare Disiloxan-Bildung. 2,2′,3,3′,4,4′,5,5′,6,6,8,8-Dodecamethyl-7-oxa-6,8-disila[3]ferrocenophan3 wurde durch Luftoxidation von 2,2′,3,3′,4,4′,5,5′,6,6,7,7-Dodecamethyl-6,7-disila[2]ferrocenophan erhaeten. Die Kristallstrukturen beider Verbindungen wurden durch Einkristall-Röntgenstrukturanalyse bestimmt (2:a=8.5330(10),b=15.610(3),c=18.774(5)Å, α=70.68(2), β=77.94(2), γ=75.150(10)°,V=2259.8(8)Å3,Z=6, Raumgruppe P\(\bar 1\),R=0.045,R w =0.044;3:a=12.388(3),b=9.924(3),c=19.136(10)Å, β=105.11(3)°,V=2271.2(15)Å3,Z=4, Raumgruppe P21/c,R=0.076,R w =0.060). Wegen der Flexibilität der Disiloxan-Brücke sind2 und3 ungespannte Moleküle.
Similar content being viewed by others
References
Hisatome M., Watanabe J., Kawajiri Y., Yamakawa K. (1990) Organometallics9: 497
See, for example: Herberhold M., Leitner P., Thewalt U. (1990) Z. Naturforsch.45b: 1503; Herberhold M., Leitner P. (1991) J. Organomet. Chem.411: 233; Herberhold M., Dörnhöfer C., Scholz A., Jin G.-X. (1992) Phosphorus Sulfur Silicon Relat. Elem.64: 161; Zanello P., Opromolla G., Casarin M., Herberhold M., Leitner P. (1993) J. Organomet. Chem.443: 199
Siemeling U., Neumann B., Stammler H.-G. (1993) Chem. Ber.126: 1311
The synthesis of2 was reported as early as 1961 by Schaaf et al. using a strategy similar to ours: Schaaf R. L., Kan P. T., Lenk C. T. (1961) J. Org. Chem.26: 1790
It is well known that strained disilanes can react with dioxygen to give disiloxanes; see, for example: Atwell W. H., Uhlmann J. G. (1973) J. Organomet. Chem.52: C21; Seyferth D., Vick S. C. (1977) J. Organomet. Chem.125: C11; Sakurai H., Kobayashi T., Nakadaira Y. (1978) J. Organomet. Chem.162: C43
Krallmann R. (1991) Thesis, Bielefeld, p. 158
It cannot be ruled out that ferrocenium ions participate in the reaction as catalyst: Tamao K., Kumada M., Takahashi T. (1975) J. Organomet. Chem.94: 367
Elschenbroich C., Salzer A. (1988) Organometallchemie, 2nd ed. Teubner, Stuttgart, p. 380; Freyberg D. P., Robbins J. L., Raymond K. N., Smart J. C. (1979) J. Am. Chem. Soc.101: 892
Wells A. F. (1991) Structural Inorganic Chemistry, 5th ed. (4th corr. repr.). Clarendon Press, Oxford, p. 1000
There has been some controversy about the reasons for this unusual behaviour; for a comprehensive discussion, see: Shambayati S., Blake J. F., Wierschke S. G., Jorgensen W. L., Schreiber S. L. (1990) J. Am. Chem. Soc.112: 697
Additional material to the structure determination can be ordered from Fachinformationszentrum Energie-Physik-Mathematik, D-76344 Eggenstein-Leopoldshafen, Federal Republic of Germany, referring to the deposition numbers CSD-57418, to the names of the authors and the citation of the paper.
Ibers J. A., Hamilton W. C. (eds.) (1974) International Tables for X-ray Crystallography, Vol. IV. Kynoch Press, Birmingham
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Siemeling, U., Krallmann, R., Jutzi, P. et al. [3]Ferrocenophanes with a tetramethyldisiloxane bridge: Synthesis and molecular structure. Monatsh Chem 125, 579–586 (1994). https://doi.org/10.1007/BF00811851
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00811851