Summary
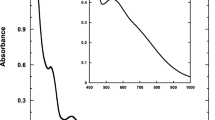
The complex formation between Gd(III) tetraphenylporphyrin or free-base tetraphenylporphyrin and vitamin E have been studied by spectrophotometric titration in chloroform and cyclohexane solutions. It has been shown that tetraphenylporphyrin and its gadolinium complex form 1:1 molecular complexes with vitamin E. The absorption spectra of titrated porphyrins contain well defined isosbestic points. The equilibrium constants were found using curve fitting procedure. The observed interactions are stronger for metallated than for non-metallated porphyrin and in less polar than in polar solvents.
Zusammenfassung
Die Ausbildung von Molekülverbindungen zwischen Gd(III)-Tetraphenylporphyrin oder der freien Base und Vitamin E wurden mittels spektrophotometrischer Titration in Cyclohexan und Chloroform untersucht. Es wird gezeigt, daß Tetraphenylporphyrin und sein Gadoliniumkomplex mit Vitamin E Molekülverbindungen äquimolarer Zusammensetzung bilden. Die Absorptionsspektren der titrierten Porphyrine zeigen gut definierte isosbestische Punkte. Die Gleichgewichtskonstanten wurden unter Zuhilfenahme einescurve-fitting-Algorithmus ermittelt. Die beobachteten Wechselwirkungen sind stärker für metalliertes als für nichtmetalliertes Porphyrin und in unpolaren als in polaren Lösungsmitteln.
Similar content being viewed by others
References
Horrocks D. W., Wong C. P. W. (1976) J. Am. Chem. Soc.98: 7157
Williams R. J. P. (1982) Struct. Bonding (Berlin) (1982)50: 79
Bünzli J. C. G., Choppin G. R. (1989) Lanthanide Probes in Life, Chemical and Earth Sciences, Theory and Practice. Elsevier, Amsterdam
Winkleman J., Slater G., Grossman J. (1967) J. Cancer Res.27: 2060
Hambright P., Fawwaz R., Valk P., McRae J., Bearden A. J. (1975) Bioinorg. Chem.5: 87
Marzola P., Cannistraro S. (1987) Physiol. Chem. Medical NMR19: 279
Wei C. C., Hsu W. S., Tominaga Y., Tsasi J. C., Chai C. Y. (1993) Nuclear Instrum. and Meth. in Phys. Res.B75: 195
Haye S., Hambright P. (1991) J. Coord. Chem.22: 315
Lomova T. N., Andrianova L. G., Berezin B. D. (1988) Koord. Khim.14: 459
Lavallee D. K. (1985) Coord. Chem. Rev.61: 55
Mauzerall D. (1965) Biochem.4: 1801
Hill H. A. O., Macfarlane A. J., Williams R. J. P. (1969) J. Chem. Soc. (A) 1704
Krishnan V. (1984) Proc. Indian Acad. Sci. (Chem. Sci.)93: 767
Pullman B., Pullmman A. (1964) In: Quantum Biochemistry, chapter IX. Interscience, New York
Heathcote J. G., Hill G. J., Rothwell P., Slifkin M. A. (1968) Biochim. Biophys. Acta153: 13
Sidorov A. N. (1974) Biofizyka19: 45
Mehdi S. H., Brisbin D. A., McBryde W. A. E. (1975) J. Solution Chem.4: 497
Horrocks W. D. W., Hove E. G. (1978) J. Am. Chem. Soc.100: 4386
Wong C. P. (1982) Inorg. Synth.22: 156
Radzki S., Krausz P., Giannotti C. (1987) Inorg. Chim. Acta.138: 139
Suzuki N., Saitoch K., Shibata Y. (1990) J. Chromatogr.504: 179
SigmaPlot. version 5.01. (1986–1982) Jandel Corporation
Beck M. T. (1970) Chemistry of Complex Equilibria. Van Nostrand Reinhold Company, London, p. 93
Sigma Plot. Scientific Graphic Software. User's Manual (1992) Jandel Scientific Corporation, Corte Madera, CA
White W. J. (1978) In: Dolphin D (ed.) The porphyrins, vol. 5. Academic Press, New York, p. 303
Burton G. W., Ingold K. U. (1986) Acc. Chem. Res.19: 194
Foster R. (1969) Organic Charge Transfer Complexes. Academic Press, London
Radzki S., Giannotti C. (1993) Inorg. Chim Acta205: 213
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Radzki, S., Krausz, P. Molecular complex formation between gadolinium(III) tetraphenylporphyrin and vitamin E. Monatsh Chem 126, 51–59 (1995). https://doi.org/10.1007/BF00811756
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00811756