Summary
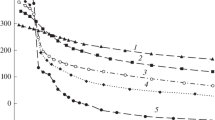
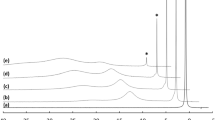
The conditional protonation constants (μ=0.1) for 2,2′:6′,2″-terpyridine, logK 1=4.93, logK 2=3.69, were determined by thepH-metric method. The compositions of complexes of Ag2+ and Ag+ ions with 2,2′:6′,2″-terpyridine (tp) were studied and equilibria of the complex formation process were described. The values of conditional complex formation constants are as follows: for Ag(tp) +2 :logβ01=5.79, logβ02=9.68, for Ag(tp) 2+2 :logβ02=25.31, while the conditional constant of the Ag(tp)NO3 precipitate formation is:K SO=2.45·104. Using coulometric and chronovoltamperometric measurements, the redox systems being formed in the complex solutions of Ag(II) and Ag(I) were determined and described including their formal potentials.
Zusammenfassung
Mit Hilfe derpH-metrischen Methode wurden die konditionalen Protonationskonstanten (μ=0.1) von 2,2′:6′,2″-Terpyridin bestimmt: logK 1=4.93, logK 2=3.69. Es wurde auch die Zusammensetzung der Komplexe von Ag(II) und Ag(I) mit 2,2′:6′,2″-Terpyridin(tp) bestimmt sowie die Gleichgewichte der komplexbildung beschrieben. Die Werte der Konditionalkomplexbildungskonstanten sind: für Ag(tp) +2 :logβ01=5.79, logβ02=9.68, für Ag(tp) 2+2 :logβ02=25.31 und für das Löslichkeitsprodukt Ag(tp)NO3:K −1SO =4.08·10−5. Die in Komplexlösungen von Ag(II) und Ag(I) vorliegenden Redoxsysteme wurden mittels cyclischer Voltametrie und Coulometrie untersucht und die Formalpotentialwerte dieser Systeme in Wasser bestimmt.
Similar content being viewed by others
References
Spravochnik khimika (1964) Khimia, Vol. 3. Moskva, p. 805
Usmani M. A. A., Scaife D. B. (1975) Pakistan J. Sci. Ind. Res.18: 214
Usmani M. A. A., Scaife D. B. (1976) Pakistan J. Sci. Ind. Res.19: 4
Talarmin J., Le Mest Y., L'Her M., Courtot-Coupez J. (1982) Electrochim. Acta27: 47
Talarmin J., Le Mest Y., L'Her M., Courtot-Coupez J. (1984) Electrochim. Acta29: 957
Talarmin J., Le Mest Y., L'Her M., Courtot-Coupez J. (1984) Electrochim. Acta29: 1037
Talarmin J., Courtot-Coupez J. (1984) Electrochim. Acta29: 967
Courtot-Coupez J., L'Her M. (1969) Bull. Soc. Chim. de France: 675
Ignaczak M., Grzejdziak A. (1989) Polish J. Chem. (in press)
Ignaczak M., Grzejdziak A., Abraszewski A. (1982) Polish J. Chem.56: 609
Ignaczak M., Grzejdziak A. (1986) Monatsh. Chem.117: 1123
Ignaczak M., Grzejdziak A. (1986) Polish J. Chem.60: 347
Ignaczak M., Grzejdziak A. (1988) Monatsh. Chem.119: 71
Ignaczak M., Grzejdziak A. (1984) Monatsh. Chem.115: 943
Prasad J., Peterson N. C. (1969) Inorg. Chem.8: 1622
Benton D. J., Moore P. (1973) J. Chem. Soc. Dalton: 399
Morgan G. T., Burstall F. H. (1934) J. Chem. Soc.: 1498; (1937) J. Chem. Soc.: 1649
Harris C. M., Lockyer T. N. (1970) Aust. J. Chem.23: 673
Murtha D. P., Walton R. A. (1973) Inorg. Nucl. Chem. Lett.9: 819
Gagliardi E., Presinger P. (1964) Mikrochim. Acta [Wien]1964: 1175
Kauffman Th., Koenig J., Wolterman A. (1976) Ber.109: 3864
Thorpe W. G., Kochi J. K. (1971) J. Inorg. Nucl. Chem.33: 3958
Gatehouse B. M., Livingstone S. E., Nyholm R. S. (1957) J. Am. Chem. Soc.79: 4222
Murtha D. P., Walton R. A. (1973) Inorg. Chem.12: 368, 1278
Rossoti F. J. C., Rossotti H., Whewell R. J. (1971) J. Inorg. Nucl. Chem.33: 2051
Kirschenbaum L. J., Ambrus J. A., Atkinson G. (1973) Inorg. Chem.12: 283
Chatterjee B., Syamal A. (1970) J. Indian Chem. Soc.47: 1021
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ignaczak, M., Grzejdziak, A. & Olejniczak, B. Complexing equilibria and redox potentials of the Ag(II)/Ag(I) system in the presence of 2,2′:6′,2″-terpyridine in water. Monatsh Chem 120, 515–527 (1989). https://doi.org/10.1007/BF00810838
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00810838