Summary
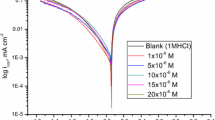
Measurements of the corrosion rate of aluminium in 2N HCl at 27°C with and without addition of phenyl semicarbazide derivatives (10−3−10−5 mol/l) were performed. The adsorption of these compounds was elucidated. Results show that phenyl semicarbazide derivatives are adsorbed on the aluminium surface according to the Frumkin isotherm. From the adsorption isotherm some thermodynamic data for the adsorption process (ΔG° ads . andf) are calculated and discussed.
Zusammenfassung
Es wurden Messungen der Korrosionsgeschwindigkeit von Aluminium in 2N HCl bei 27°C mit und ohne Zusatz von Phenylsemicarbaziden (10−3−10−5 mol/l) durchgeführt. Es wurde die Adsorption dieser Verbindungen untersucht, wobei sich zeigte, daß die Adsorption der Phenylsemicarbazidderivate der Frumkin-Isotherme gehorcht. Aus der Isotherme wurden einige thermodynamische Parameter des Adsorptionsprozesses (ΔG° ads. ,f) berechnet.
Similar content being viewed by others
References
Iofa Z. A., Medvedeva A. A. (1940) Dokl. Akad. Nauk SSSR69: 213
Mayanna S. M. (1975) J. Electrochem. Soc.122: 251
Mayanna S. M., Setty T. H. V. (1975) Corros. Sci.15: 627
Anmed M. F., Mayanna S. M., Pushpanaden F. (1975) In: 6th International Congress on Metallic Corrosion. University of Sydney, Australia
Frumkin A. N. (1925) Z. Phys. Chem.116: 466
Mann C. A., Lauer B. E., Huttin C. T. (1936) Ind. Eng. Chem.28: 159
Ciao S.-J., Mann C. A. (1947) Ind. Eng. Chem.39: 910
Bockris J. O. M., Green M., Swinkels D. A. J. (1964) J. Electrochem. Soc.111: 736
Szklarska-Smialowska Z., Kaminski M. (1971) Corros. Sci.11: 843
Damaskin W. W., Pietrij O. A., Batrakow W. W. (1968) Adsorpcja Organiczeskich Sojedinienijna Elektrodach. Moskow
Cornet I., Fuerstenau D. W. (1970) U.S. Clearing House Fed. Sci. Tech. Infarm. AD 1970, No. 709468
Murakawa T., Nagura S., Hackerman N. (1967) Corros. Sci.7: 79
Hackerman N., Shavely E. S., Payna J. S., Jr. (1966) J. Electrochem. Soc.113: 677
Murakawa T., Kato T., Nagura S., Hackerman N. (1968) Corros. Sci.8: 483
Rudresh H. B., Mayanna S. M. (1977) J. Electrochem. Soc.124: 340
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fouda, A.EA.S., Mostafa, H.A. & Abu-Elnader, H.M. Phenyl semicarbazide derivatives as corrosion inhibitors for aluminium in hydrochloric acid solution. Monatsh Chem 120, 501–507 (1989). https://doi.org/10.1007/BF00810836
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00810836