Summary
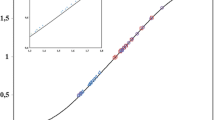
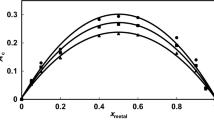
Investigations on the composition of the molybdate(II)-3-hydroxyflavone complex and its stability constant have been carried out in 70% ethanolic solutions at room temperature (20°C), in the presence of a buffer atpH 6.30±0.05 and ionic strength 0.015. It has been found that a complex [MoO3(C15H9O3) 2−2 ] whose stability constant ranges from 15.13 atpH 6.25 to 13.02 atpH 7.00 is formed. Conditions are given for the determination of 3-hydroxyflavone by means of the reaction of complex formation in 50% ethanol atpH 6.60±0.05 and an ionic strength of 0.0225.
Zusammenfassung
Die Untersuchungen der Zusammensetzung des Komplexes und seiner Stabilitätskonstante wurden in 70% Ethanol, bei Raumtemperatur (20°C), in Anwesenheit von Puffer beipH=6.30±0.05 und der Ionenstärke 0.015 ausgeführt. Es wird der Komplex [MoO3(C15H9O3) 2−2 ] gebildet, die Stabilitätskonstanten reichen von 15.13 beipH 6.25 bis zu 13.02 beipH 7.00. Es wurden die Bedingungen zur quantitativen Bestimmung von 3-Hydroxyflavon mittels des Molybdenkomplexes in 50% Ethanol, beipH=6.60±0.05 und der Ionenstärke 0.0225 ermittelt.
Similar content being viewed by others
References
Katyal M. (1968) Talanta15: 95
Katyal M., Prakash S. (1977) Talanta24: 367
Nevskaya E. M., Nazarenko V. A. (1972) Zh. Analit. Khim.27: 1696
Schiller K., Thilo E. (1959) Z. Anorg. Allgem. Chem.8: 718
Velašević K., Radović Z., Djukanović A. (1979) Arch. Pharm. (Beograd)29: 239
Radović Z., Malešev D. (1983) Arch. Pharm. (Beograd)33: 147
Malešev D., Radović Z. (1989) Pharmazie44: 496
Irving H., Pierce T. B. (1959) J. Chem. Soc.: 2565
Yoe J. H., Jones A. L. (1944) Ind. Eng. Chem., Anal. Ed.16: 111
Šušić M., Veselinović D., Sužnjević D. (1964) Bull. Soc. Chim. Beograd29: 121
Savčenko G. S. (1955) Him. Red. Elementov2: 68
Bettinger P. J., Tyree S. Y. (1957) J. Am. Chem. Soc.79: 3355
Inczédy J. (1976) Analytical Applications of Complex Equilibria. Wiley, New York
Veselinović D., Šušić M. (1965) Bull. Soc. Chim. Beograd30: 79
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Malešev, D., Radović, Z. & Jelikić-Stankov, M. Investigation of the molybdate(II)-3-hydroxyflavone complex. Monatsh Chem 122, 429–434 (1991). https://doi.org/10.1007/BF00809794
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00809794