Abstract
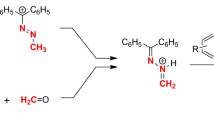
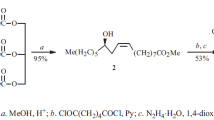
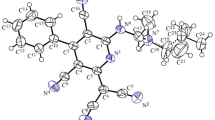
Model compound5 was prepared by acid catalyzedintramolecular nucleophilic addition of the alcohol (Z)-4. In comparison to theintermolecular addition productl-2, differences in structure and reactivity could be determined by X-ray crystal structure analysis and1H-NMR-spectroscopic investigations. The interpretation of these differences are based on the assumption of stereoelectronic advantages in the intermolecular adductl-2. Its methoxy group was found to beperiplanar with respect to the π-(NCO)-plane of the lactam unit enabeling efficient delocalization of the amide lone pair to the σ*-orbital of the C-OMe-bond. The orientation of the corresponding C-O-bond in5 is fixed by the rigid structure of the bicyclic ring system and can be classified assynclinal. Consequentlyl-2 eliminates methanol very easily under acidic or high temperature conditions, whereas5 is a stable compound.
In the stereoelectronically favoured adductl-2 n → σ*-delocalization increases elimination reactivity on the one hand and thermodynamic stability on the other hand. Therefore adducts of this kind may be good candidates for biological regulators—a matter of interest with regard to the protein-chromophore interaction in biliproteins.
Similar content being viewed by others
Literatur
Grubmayr K, Wagner GU (1988) Monatsh Chem 119: 793
Kirby AJ (1983) The anomeric effect and related stereoelectronic effects at oxygen. Springer, Berlin Heidelberg New York Tokyo
Deslongchamps P (1983) Stereoelectronic effects in organic chemistry. Pergamon Press, Oxford (Organic Chemistry Series, vol 1)
Falk H, Zrunek U (1983) Monatsh Chem 114: 983
Medinger W (1987) Dissertation, Universität Wien
Wiederkehr R (1968) Dissertation, ETH Zürich, Diss. Nr. 4239
Falk H, Zrunek U (1984) Monatsh Chem 115: 101
Grüning B, Holze G, Jenny TA, Nesvadba P, Gossauer A, Ernst L, Sheldrick WS (1985) Helv Chim Acta 68: 1771
Pfaltz A, Jaun B, Fässler A, Eschenmoser A, Jaenchen R, Gilles HH, Diekert G, Thauer RK (1982) Helv Chim Acta 65: 828
Fässler A, Kobelt A, Pfaltz A, Eschenmoser A, Bladon C, Battersby AR, Thauer RK (1985) Helv Chim Acta 68: 2287
Grubmayr K, Kapl G (1988) Monatsh Chem 119: 605
Dunitz JD (1979) X-Ray analysis and structure of organic molecules. Cornell University Press, London
Jones PG, Kirby AJ (1979) J Chem Soc Chem Comm 1979: 288
Brown KL, Down GJ, Dunitz JD, Seiler P (1982) Acta Cryst B 38: 1241
Cullen DL, van Opdenbosch N, Meyer EF Jr, Smith KM, Eivazi F (1982) J Chem Soc Perkin II 1982: 307
Bonfiglio JV, Bonnett R, Buckley DG, Hamzetash D, Hursthouse MB, Malik KMA, Naithani SC, Trotter J (1982) J Chem Soc Perkin Trans I 1982: 1291
Kalinowski H-O, Berger S, Braun S (1984)13C-NMR-Spektroskopie. G Thieme, Stuttgart
Lasne M-C, Ripoll J-L, Thuillier A (1982) J Chem Res (S) 1982: 214
Speckamp WN, Hiemstra H (1985) Tetrahedron 41: 4367
Wray V, Gossauer A, Grüning B, Reifenstahl G, Zilch H (1979) J Chem Soc Perkin Trans II 1979: 1558
Falk H, Müller N, Vormayr G (1984) Org Magn Reson 22: 576
Falk H, Grubmayr K, Müller N, Vormayr G (1985) Monatsh Chem 116: 53
Moss GP (1987) Pure Appl Chem 59: 779
Grubmayr K (1982) Monatsh Chem 113: 1073
Allen FH, Kennard O, Taylor R (1983) Acc Chem Res 16: 146
Wagner GU (1987) Dissertation, Karl-Franzens-Universität Graz
German G, Main P, Woolfson MM (1971) Acta Cryst A 27: 368
Sheldrick GM (1976) Shelx 76, a program for crystal structure determination. University of Cambridge, England
Motherwell S (1976) Program Pluto. University of Cambridge, England
Johnson CK (1976) Ortep report ORNL 5138. Oak Ridge National Laboratory, Oak Ridge, Tennessee, U.S.A.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Grubmayr, K., Wagner, U.G. Zur intramolekularen nucleophilen Addition von 2,3-Dihydrodipyrrin-1(10H)-onen. Monatsh Chem 119, 813–831 (1988). https://doi.org/10.1007/BF00809693
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00809693