Abstract
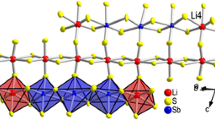
Black, fibre-like crystals of Tl2SnS3 were obtained from the melt using high-purity elements. Tl2SnS3 is monoclinic, space group C 2/m witha=23.03 (1),b=3.834 (1),c=7.379 (3) Å, β=94.07 (5)°;Z=4. The crystal structure was determined from single crystal diffractometer intensity data and refined to a conventionalR of 0.127 for 724 observed reflections. The crystal structure is of a new type. It is characterized by infinite chains, ∞1 -[SnS 2−3 ], formed by cornersharing SnS4-tetrahedra. These chains run along [010], their translation period comprises one tetrahedron (“Einerketten”). The average Sn — S-distance is 2.397 Å. The Tl+-ions separating the thiostannate-chains have different sulfur coordination. The coordination figure of Tl(1) is a bicapped trigonal prism, Tl(2) is in the center of an irregular cube. A comparison of the thiostannate-chains with other simple chains built-up by corner-sharing tetrahedra is given.
Similar content being viewed by others
Literatur
Krebs B., Angew. Chem.95, 113 (1983).
Jumas J. C., Vermot-Gaud-Daniel F., Philippot E., Cryst. Struct. Comm.2, 157 (1973).
Jumas J. C., Philippot E., Vermot-Gaud-Daniel F., Ribes M., Maurin M., J. Solid State Chem.14, 319 (1975).
Krebs B., Schiwy W., Z. anorg. allg. Chem.398, 63 (1973).
Mark W., Lindqvist O., Jumas J. C., Philippot E., Acta Cryst.B 30, 2620 (1974).
Krebs B., Pohl S., Schiwy W., Z. anorg. allg. Chem.393, 241 (1972).
Jumas J. C., Philippot E., Maurin M., J. Solid State Chem.14, 152 (1972).
Klepp K. O., Eulenberger G., Z. Naturforsch.39 b, 705 (1984).
Eulenberger G., Z. Naturforsch.36 b, 687 (1981).
The X-RAY-System (1976). Techn. Rep. TR-446, Computer Science Center, Univ. of Maryland, College Park, Maryland, U.S.A.
Cromer D. T., Mann J. B., Acta Cryst.A 24, 321 (1968).
International Tables for X-Ray Crystallography, Vol. IV. Birmingham: The Kynoch Press. 1974.
Liebau F., Naturwiss.49, 41 (1962).
Schiwy W., Blutau Cl., Gäthje D., Krebs B., Z. anorg. allg. Chem.412, 1 (1975).
Shannon R. D., In: Structure and Bonding in Crystals (Navrotsky A., O'Keefe M., Hrsg.), Vol. II, S. 53. New York: Academic Press. 1981.
Brink C., MacGillavry C. H., Acta Cryst.2, 158 (1949).
Brink C., Stenfert-Kroese H. A., Acta Cryst.5, 433 (1952).
Brink C., vanArkel A. E., Acta Cryst.5, 506 (1952).
Pauling L., In: The Nature of the Chemical Bond. Ithaca: Cornell Univ. Press. 1940.
Ribes M., Olivier-Fourcade J., Philippot E., Maurin M., Rev. Chim. Min%er.9, 757 (1972).
Schartau W., Hoppe R., Z. anorg. allg. Chem.408, 60 (1974).
Völlenkle H., Z. Krist.154, 77 (1981).
McDonald W. S., Cruickshank D. W. J., Acta Cryst.22, 37 (1967).
Cruickshank D. W. J., Kálman A., Stephens J. S., Acta Cryst.B 34, 1333 (1978).
Grey I. E., Steinfink H., Inorg. Chem.10, 691 (1971).
Iglesias J. E., Pachali K. E., Steinfink H., J. Solid State Chem.9, 6 (1974).
Sommer H., Hoppe R., Z. anorg. allg. Chem.443, 201 (1978).
Hong H. Y., Steinfink H., J. Solid State Chem.5, 93 (1972).
v. Schnering H. G., Hoppe R., Z. anorg. allg. Chem.312, 99 (1961).
Jaulmes S., Houenou P., Mat. Res. Bull.15, 911 (1980).
Ajavon A.-L., Eholie R., Piffard Y., Tournoux M., Rev. Chim. Minér.20, 421 (1983).
Yvon K., Jeitschko W., Parthé E., J. Appl. Cryst.10, 73 (1977).
Author information
Authors and Affiliations
Additional information
Herrn Prof. Dr.Karl Schlögl zum 60. Geburtstag gewidmet.
Rights and permissions
About this article
Cite this article
Klepp, K.O. Tl2SnS3 — ein Thiostannat mit ∞1 -[SnS 2−3 ]-Ketten. Monatsh Chem 115, 1133–1142 (1984). https://doi.org/10.1007/BF00809344
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00809344