Abstract
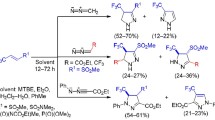
TheGrignard reagents3 attack the azo group of the α-arylazoalkylisocyanate2 in the manner of a nucleophilic addition; by involving the geminally situated isocyanate function the heterocyclic anions6 are formed and afford the 1-N-substituted triazolidinones7 upon hydrolytic work-up. Besides, some portion of the anions6 acting as a N-nucleophile adds to another isocyanate2 and gives rise to the adducts8. Furthermore, the use of ethylmagnesiumbromide also causes the reduction of the azo group of2 as was evidenced by the additional isolation of the 1-N-unsubstituted triazolidinones1 and15; the latter appears to be the ring tautomer of the primarily formed semicarbazone14. Only with phenylmagnesiumbromide3d the direct adduct to the isocyanate function of2—the benzamide derivative4d—was obtained as a minor product.
Similar content being viewed by others
Literatur
Schantl J. G., Monatsh. Chem.101, 568 (1970).
Schantl J. G.,Hebeisen P.,Minach L., Synthesis1984, 315.
Schantl J. G., Hebeisen P., Sci. Pharm.51, 379 (1983).
Richter R., Ulrich H., Synthesis and Preparative Applications of Isocyanates; in: The Chemistry of Cyanates and Their Thio Derivatives (Patai S., ed.), 2. Bd., S. 773. Chichester: J. Wiley. 1977.
Nützel K., Organo-Magnesium-Verbindungen, inHouben-Weyl: Methoden der organischen Chemie, Bd. 13/2a. Stuttgart: G. Thieme. 1973. a) S. 297, b) S. 393, c) S. 396, d) S. 519.
Gilman H., Pickens R. M., J. Amer. Chem. Soc.47, 2406 1925).
Beyer H., Kröger C.-F., Zander M., Chem. Ber.88, 1233 (1955).
Colonna M., Risaliti A., Gazz. chim. ital.86, 698 (1956).
Risaliti A., Ann. Chim. (Rome)87, 120 (1957);
Risaliti A., Bozzini S., ibid.54, 685 (1964);
Risaliti A., Stener A., ibid.57, 3 (1967);
ibid.58, 169 (1968).
Risaliti A., Bozzini S., Stener A., Tetrahedron25, 143 (1969);
Bozzini S., Risaliti A., Stener A., Tetrahedron26, 3927 (1970).
Bozzini S., Gratton S., Risaliti A., Stener A., Calligaris M., Nardin G., J. Chem. Soc., Perkin Trans.I 1977, 1377;
Bozzini S., Gratton S., Pellizer G., Risaliti A., Stener A., J. Chem. Soc., Perkin Trans. I1979, 869.
Jochims J. C., Abu-Taka A., Chem. Ber.109, 154 (1976).
Gstach H.,Schantl J. G., unveröffentlicht.
Holm T., Crossland I., Acta Chem. Scand.B 33, 421 (1979).
Schildknecht H., Hatzmann G., Liebigs Ann. Chem.724, 226 (1969).
Anteunis M., Borremans F., Tadros W., Zaher A. H. A., Gliobrial S. S., J. Chem. Soc., Perkin Trans. I1972, 616.
Tsuge O., Kanemasa S., Bull. Chem. Soc. Japan47, 2676 (1974).
Pilgram K., Skiles R. D., Pollard G. E., J. Heterocycl. Chem.13, 1257 (1976).
Gadzhiev G. Yu., Dzhalilov E. Yu., Samitov Yu. Yu., Nematollahi J., Zh. Org. Khim.17, 1784 (1981); Chem. Abstr.95, 203899j (1981).
Author information
Authors and Affiliations
Additional information
Herrn emer. Prof. Dr.H. Bretschneider zum 80. Geburtstag gewidmet.
Rights and permissions
About this article
Cite this article
Schantl, J.G., Gstach, H. Geminale Azo- und Heteroelement-Funktionen, I: Einwirkung vonGrignard-Reagentien auf 1-(4-Chlorphenylazo)-1-methylethylisocyanat. Monatsh Chem 116, 1051–1064 (1985). https://doi.org/10.1007/BF00809197
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00809197