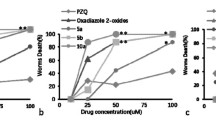
Summary
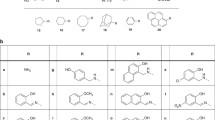
Interaction of ethyl 2-acetamido-5-bromothiazole-4-carboxylate (2) with 2-methyl-5-chlorothiophenol (3) afforded the thioether4, which was hydrolysed to the corresponding carboxylic acid5. Attempted cyclization of5 to6 yielded the decarboxylated product7. On the other hand, interaction of 2-acetamido-5-bromothiazole (9) with thiosalicylic acid (10) yielded the thioether11, which was cyclized to compound12. Acid hydrolysis of12 yielded the amino derivative13, which was reacted with certain selected alkyl halides using sodium hydride to afford compounds14–18.
Zusammenfassung
Die Reaktion von Ethyl 2-Acetamido-5-bromthiazol-4-carboxylat (2) mit 2-Methyl-5-chlorthiophenol (3) ergab den Thioether4, der zur entsprechenden Carbonsäure5 hydrolysiert wurde. Die versuchte Cyclisierung von5 zu6 ergab das Decarboxylierungsprodukt7. Andererseits ergab die Reaktion von 2-Acetamido-5-bromthiazol (9) mit Thiosalizylsäure (10) den Thioether11, der zu Verbindung12 cyclisiert werden konnte. Saure Hydrolyse von12 ergab das Aminoderivat13, das mit geeigneten Alkylhalogeniden unter Verwendung von Natriumhydrid zu den Verbindungen14–18 führte.
Similar content being viewed by others
References
Schmidt P., Wilhelm M. (1966) Angew. Chem. Int. Ed.5: 857
Werbel L. M., Battaglia J. R. (1971) J. Med. Chem.14: 10
Lin Y., Hulbert P. B., Bueding E., Robinson C. H. (1974) J. Med. Chem.17: 835
Islip P. J., Closier M. D., Neville M. C. (1974) J. Med. Chem.17: 207
Kikuth W., Gonnert R. (1948) Ann. Trop. Med. Parasitol.42: 256
Rosi D., Peruzotti G., Dennis D. A., Berberian D. A., Freele H., Archer S. (1965) Nature (London)208: 1005
Archer S., Rochester L. B., Jackman M. (1954) J. Am. Chem. Soc.76: 588
Elslager E. F. (1975) Ger. Offen 2,433,998; (1975) Chem. Abstr.83: 43318 r
Elslager E. F., Worth D., Donald E. (1976) U.S. Pat. 3,904,631; (1976) Chem. Abstr.84: 44151 p
El-Kerdawy M. M., El-Emam A. A., El-Subbagh H. I. (1986) Arch. Pharm. Res.9: 25
Sprague J. M., Lincoln R. M., Ziegler C. (1946) J. Am. Chem. Soc.68: 266
Beyerman H. C., Berben P. H., Bontekoe J. S. (1954) Rec. Trav. Chim.73: 325
Sharp T. M. (1951) J. Chem. Soc.: 2961
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
El-Kerdawy, M.M., El-Emam, A.A., El-Subbagh, H.I. et al. Synthesis of substituted 4H-thiazolo[4,5-b][1]benzothiopyran-4-ones as possible schistosomicidal agents. Monatsh Chem 120, 991–995 (1989). https://doi.org/10.1007/BF00808770
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00808770